2
answers
0
watching
355
views
28 Sep 2019
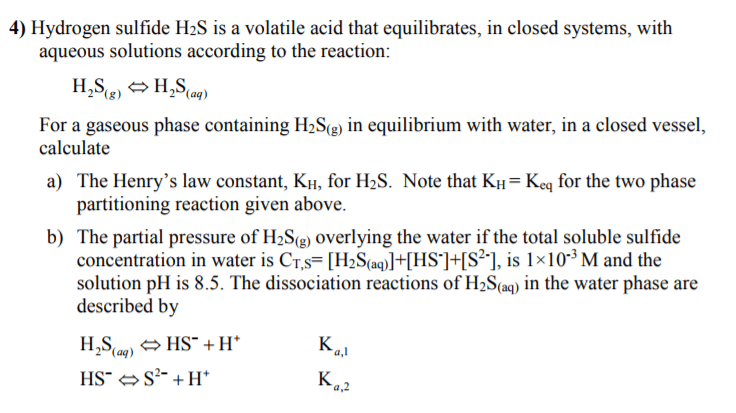
1. Iron sulfide, FeS, is a sparingly soluble salt with a Ksp of 6.3 x 10^-18. Calculate the molar solubility of iron sulfide in a solution that is saturated with hydrosulfuric acid, H2S ( [H2S] = 0.1 M) and has a pH of 2.0. Ka1 = 8.9 x 10^-8 and Ka2 = 1.2 x 10^-13.
1. Iron sulfide, FeS, is a sparingly soluble salt with a Ksp of 6.3 x 10^-18. Calculate the molar solubility of iron sulfide in a solution that is saturated with hydrosulfuric acid, H2S ( [H2S] = 0.1 M) and has a pH of 2.0. Ka1 = 8.9 x 10^-8 and Ka2 = 1.2 x 10^-13.
Read by 1 person
Deanna HettingerLv2
28 Sep 2019
Already have an account? Log in