1
answer
0
watching
98
views
10 Nov 2019
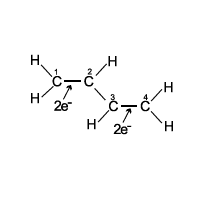
Look at thedrawing of the butadiene molecule. Which statement about thismolecule isnottrue?
By valence bond theory (and the drawing), thereare four electrons to account for in the molecule. Valence bond theory says that all the carbon atomshave hybridized sp2orbitals.
There are p orbitals (in the carbon atoms) whoseaxes actually are perpendicular to the page.
The resonance that occurs in the butadienemolecule causes the carbon-carbon bond lengths to constantlychange.

Look at thedrawing of the butadiene molecule. Which statement about thismolecule isnottrue?
| By valence bond theory (and the drawing), thereare four electrons to account for in the molecule. | |
| Valence bond theory says that all the carbon atomshave hybridized sp2orbitals. | |
| There are p orbitals (in the carbon atoms) whoseaxes actually are perpendicular to the page. | |
| The resonance that occurs in the butadienemolecule causes the carbon-carbon bond lengths to constantlychange. |
Nestor RutherfordLv2
25 Aug 2019



