1
answer
0
watching
142
views
plumkoala760Lv1
28 Sep 2019
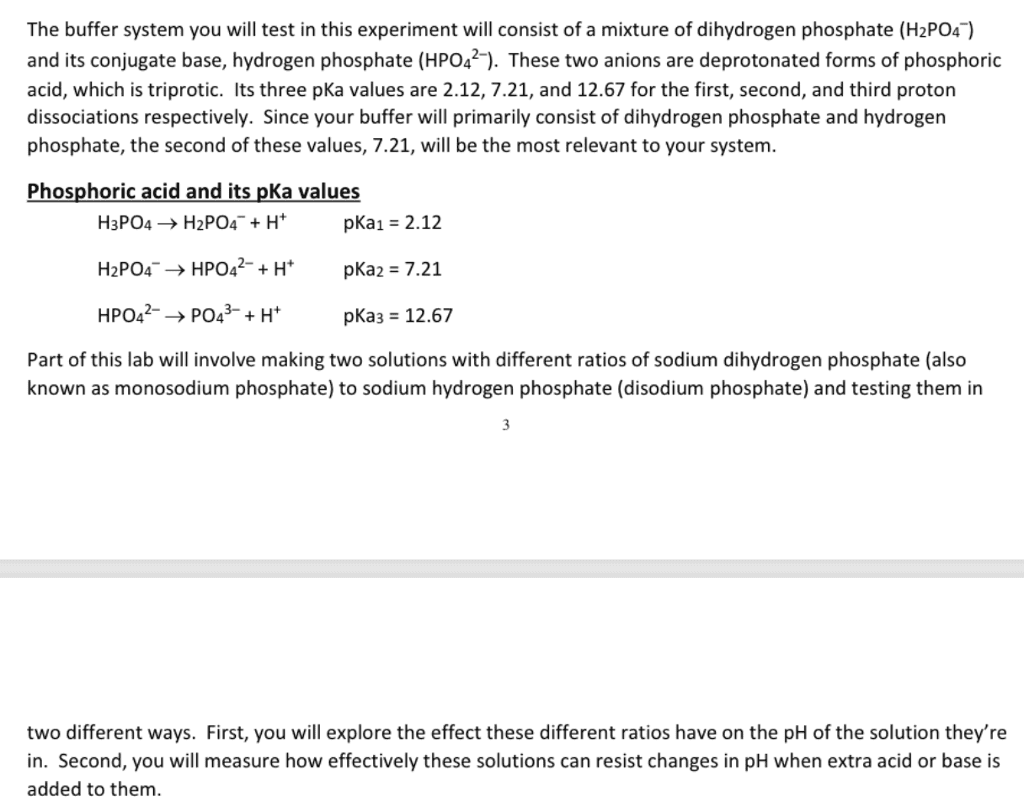
If you wanted to make a 3 liter solution of 500 mM formic acid buffer at ph 3.9 how many grams each of sodium formate (MW=68g/mol) and formic acid (MW=46g/mol) would you add... 98.6 g sodium formate and 2.3g of formic acid or 20.2 sodium formate and 9.4g formic acid OR 60.5g sodium formate and 28.1 g formic acidPlease show steps! thanks
If you wanted to make a 3 liter solution of 500 mM formic acid buffer at ph 3.9 how many grams each of sodium formate (MW=68g/mol) and formic acid (MW=46g/mol) would you add... 98.6 g sodium formate and 2.3g of formic acid or 20.2 sodium formate and 9.4g formic acid OR 60.5g sodium formate and 28.1 g formic acidPlease show steps! thanks
1
answer
0
watching
142
views
For unlimited access to Homework Help, a Homework+ subscription is required.
Reid WolffLv2
28 Sep 2019
Related textbook solutions
Basic Chemistry
5 Edition,
Timberlake
ISBN: 9780134138046
Principles of Chemistry Molecular Approach
4th Edition,
Tro
ISBN: 9780134112831
Chemistry: Structure and Properties
2nd Edition,
Tro
ISBN: 9780134293936
Principles of Chemistry Molecular Approach
3rd Edition, 2014
Tro
ISBN: 9780321971944
Chemistry: A Molecular Approach
3rd Edition,
Tro
ISBN: 9780321809247
Chemistry: A Molecular Approach
5th Edition,
Tro
ISBN: 9780134874371
Principles of Chemistry: A Molecular Approach
4th Edition,
Tro
ISBN: 9780134895741
Chemistry: The Central Science
14th Edition, 2017
Brown
ISBN: 9780134414232