1
answer
0
watching
268
views
10 Nov 2019
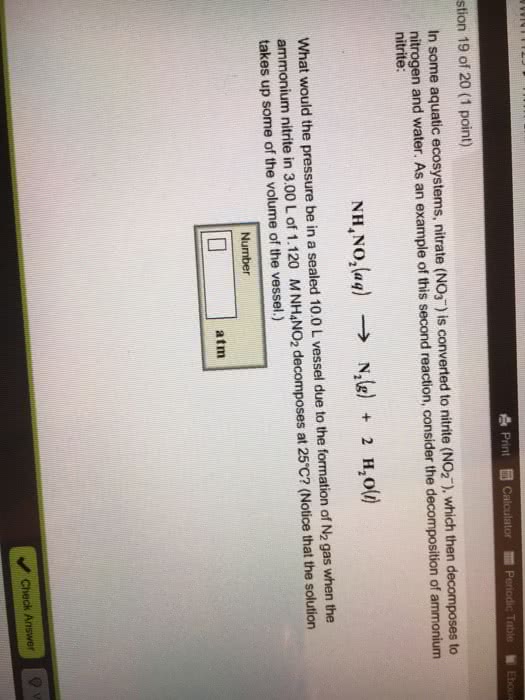
Consider the decomposition of ammonium nitrite: NH4NO2 yields to N2 + H2O. What would be the change in pressure in a sealed 10.0 L vessel due to the formation of N2 gas when the ammonium nitrite in 2.90 L of 0.990 M NH4NO2 decomposes at 25 degrees celcius? Use PV=nRT
Consider the decomposition of ammonium nitrite: NH4NO2 yields to N2 + H2O. What would be the change in pressure in a sealed 10.0 L vessel due to the formation of N2 gas when the ammonium nitrite in 2.90 L of 0.990 M NH4NO2 decomposes at 25 degrees celcius? Use PV=nRT
1
answer
0
watching
268
views
For unlimited access to Homework Help, a Homework+ subscription is required.
Nelly StrackeLv2
18 Apr 2019
Related textbook solutions
Basic Chemistry
5 Edition,
Timberlake
ISBN: 9780134138046
Principles of Chemistry Molecular Approach
4th Edition,
Tro
ISBN: 9780134112831
Chemistry: Structure and Properties
2nd Edition,
Tro
ISBN: 9780134293936
Principles of Chemistry Molecular Approach
3rd Edition, 2014
Tro
ISBN: 9780321971944
Chemistry: A Molecular Approach
3rd Edition,
Tro
ISBN: 9780321809247
Chemistry: A Molecular Approach
5th Edition,
Tro
ISBN: 9780134874371
Principles of Chemistry: A Molecular Approach
4th Edition,
Tro
ISBN: 9780134895741
Chemistry: The Central Science
14th Edition, 2017
Brown
ISBN: 9780134414232