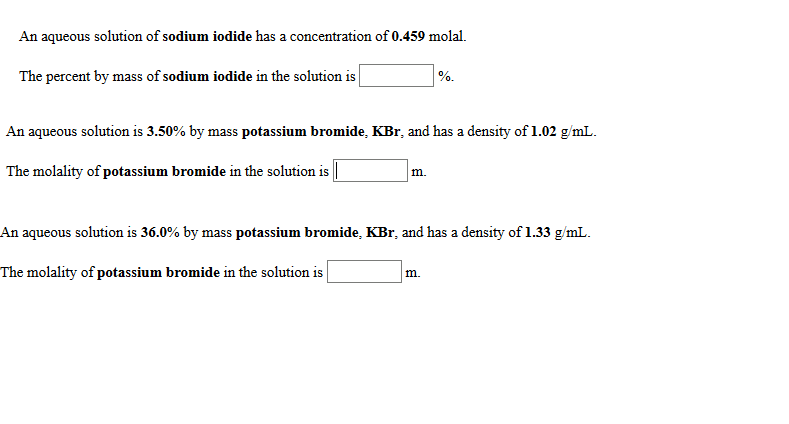
1
answer
0
watching
142
views
28 Sep 2019
In the laboratory a student combines 26.4 mL of a 0.182 M potassium bromide solution with 17.2 mL of a .329 M potassium bromide solution. What is the molarity of KBr in the final solution?
In the laboratory a student combines 26.4 mL of a 0.182 M potassium bromide solution with 17.2 mL of a .329 M potassium bromide solution. What is the molarity of KBr in the final solution?
Tod ThielLv2
28 Sep 2019