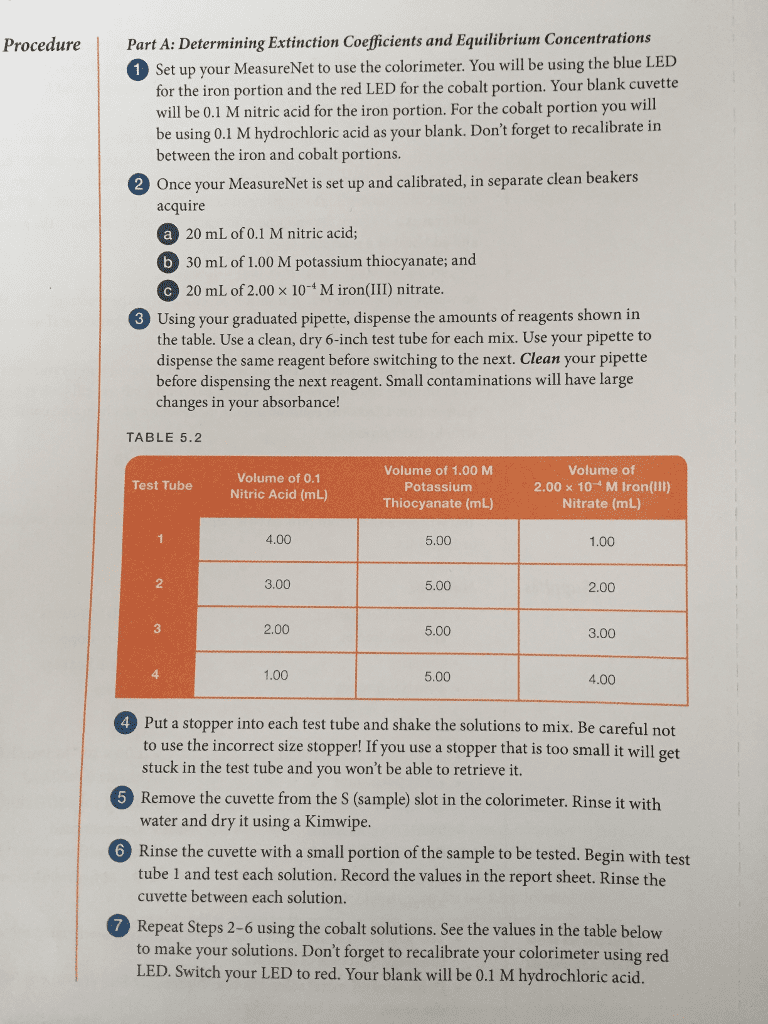
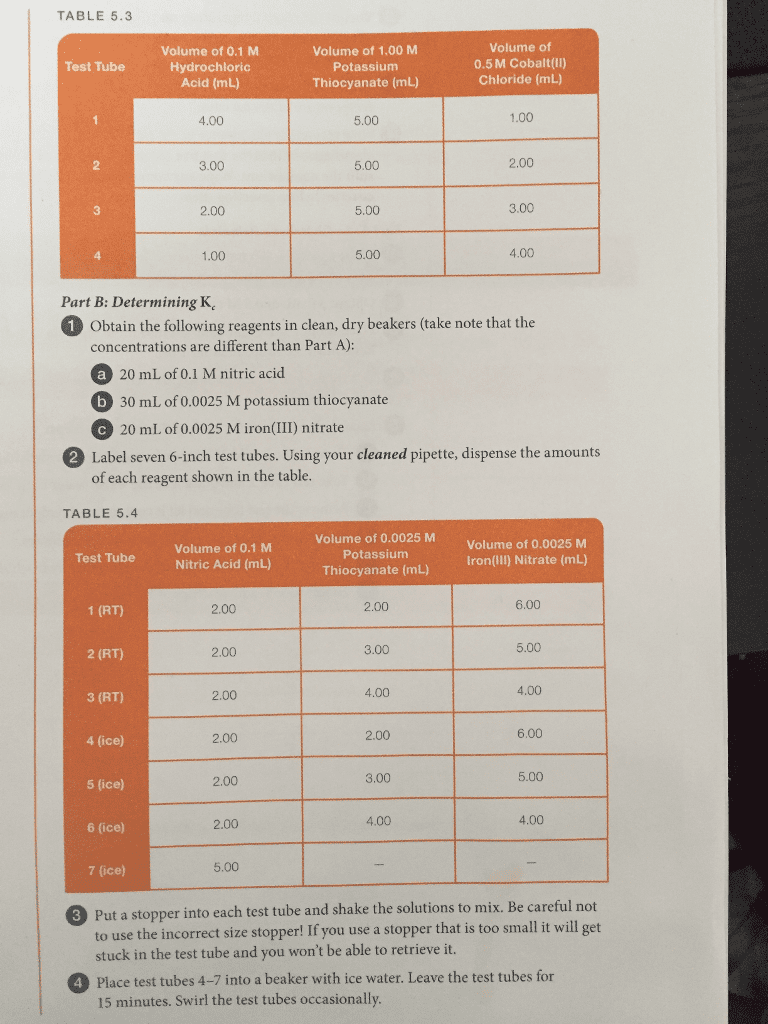
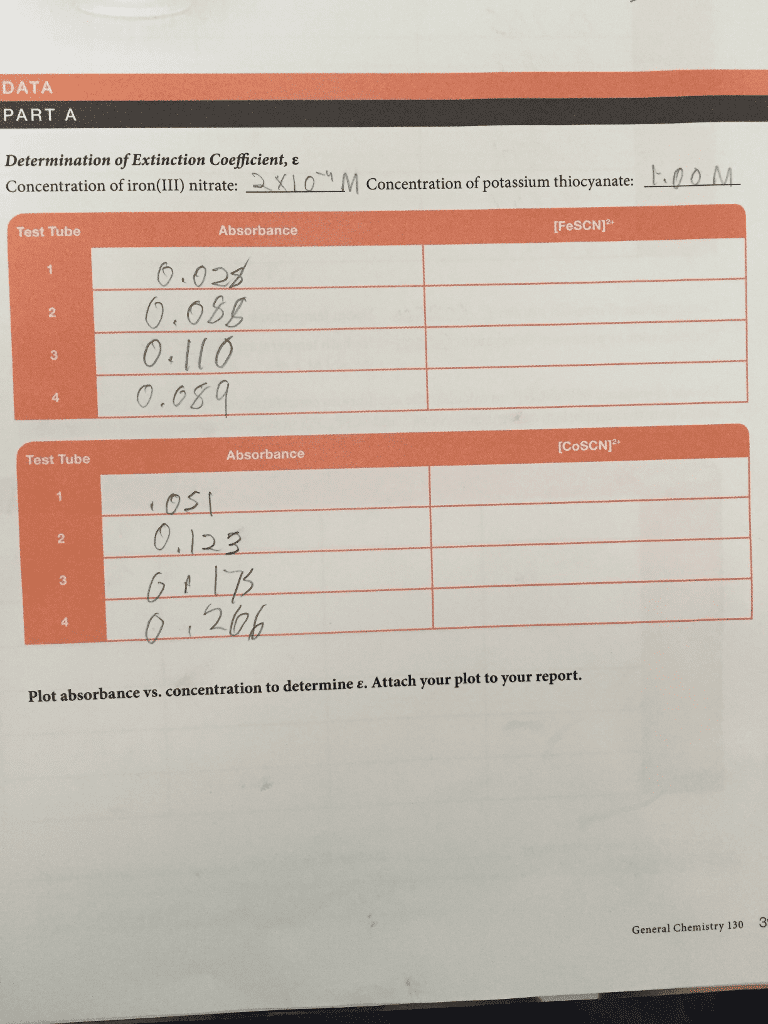
CHEM 14B Lecture Notes - Lecture 1: Ammonia, Equilibrium Constant, Partial Pressure
Document Summary
At molecular level: r forming p & reverse reaction p forming r (but at an equal rate) Under same conditions, reaction at equilibrium has same. Equilibrium constant (kc): ratio of the concentration of the product to the concentration of the reactant. Tells the relative stability of the products and reactants. Reaction at equilibrium aa + bb cc + dd. A, b, c, d: stoichiometric coefficients of blanched equation. Example: calculate kc for n2 mixed with h2 at 500 c to produce ammonia. At equilibrium: n2 = 0. 305m, h2 = 0. 324m, nh3 = Reverse rxn: 2nh3 n2 + 3h2. Kc = ([n2][h2]^3)/[nh3]^2 = 1/61. 0 = 0. 016. All r and p in same phases: homogeneous equilibrium. One of more r or p in different phase: heterogeneous equilibrium. Molar concentration of a pure substance (solid or liquid) does not change in a reaction. Solids and liquids not included in k expression. Example: ca(oh) ca+2 + 2 oh-