CHEM 1073 Lecture Notes - Lecture 5: Balance Equation
Document Summary
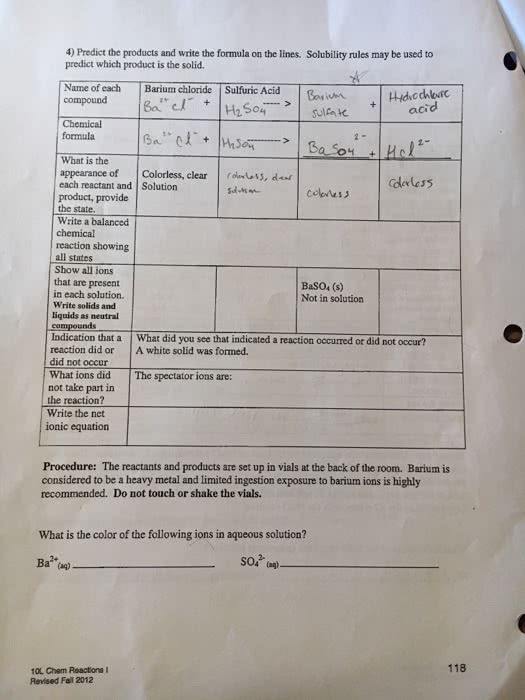
Changes that may indicate a chemical reaction. Process where a new substance is formed: color change, formation of a solid, formation of a gas, heat absorbed or produced. Show how the groupings of atoms change in a reaction. Use chemical formulas for reactants and products. Reactants: compounds and elements consumed in reaction > products: compounds and elements formed in reaction. Equations often show the states of matters for reactants and products. A balances equation shows the number of each reactant or product involved in a reaction. All atoms present in the reactants must also be present in the products. To balance: adjust coe cients of reactants and products until equation has the same number of each type of atoms on both sides. Balance equation by inspection: start with most complicated compound and elements found in only one reactant and one product, balance pure elements last.