CHEM 272 Lecture Notes - Lecture 2: Pipette, Cuvette, Standard Deviation
Document Summary
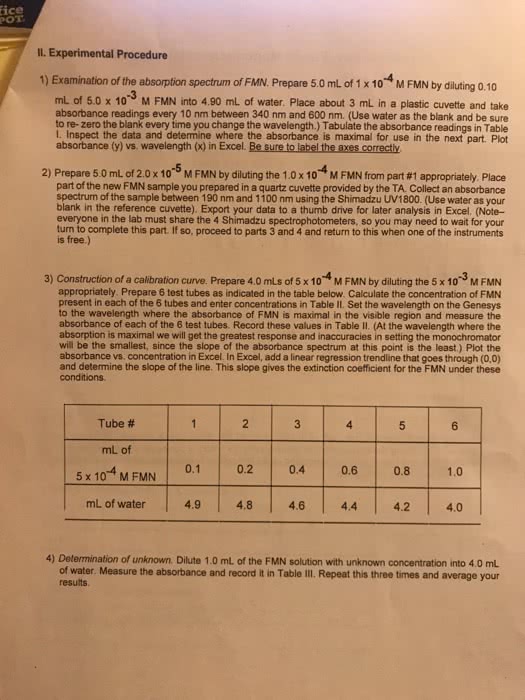
Discussion: non-linearity in a beer"s law plot can be explained by improper cuvette rinsing. In this experiment, we used the same cuvette for each trial and sample. If some of the previously measured solution remained in the cuvette when adding the new solution, this could affect the concentration. Our group went in order of increasing concentrations so if water remained in the cuvette this would dilute the new concentration causing a lower absorbance value. Another explanation could be that the material concentrations decompose. Since our experiment required us to take absorbance readings every 10 minutes, deviations could occur during any different time readings. If the unknown were measured and fell in the non-linear range, then more accurate timing could be taken, and a new cuvette could also be used. Our group used only a micropipette for measurements.