CHEM 1150 Lecture Notes - Lecture 2: Cathode Ray Tube, Chemical Change, Pauli Exclusion Principle
44 views3 pages
Document Summary
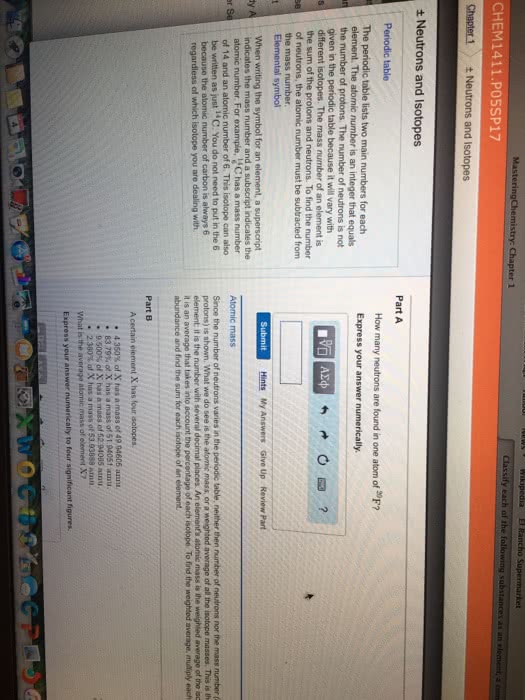
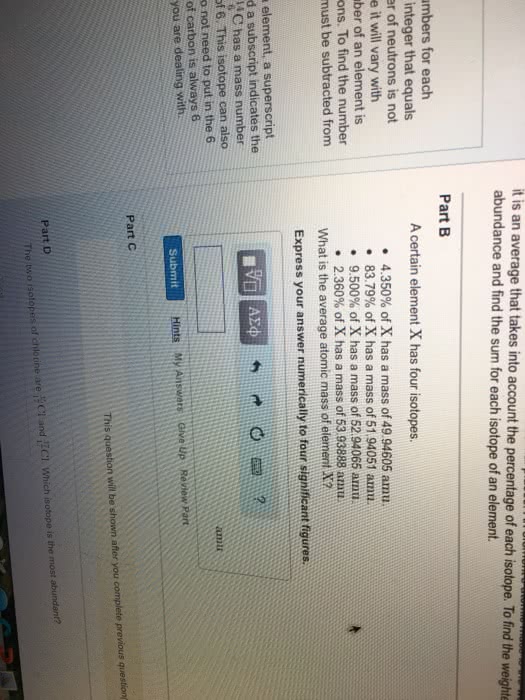
Atom= the basic structural unit of an element. Nucleus= small, dense, positively charged region in the center of the atom. Atomic number= the number of protons in the atom. Mass number= sum of the number of protons and neutrons. Number of neutrons= mass number - number of protons. Isotopes= atoms of the same element having different masses. Atomic mass= the weighted avg. of the masses all the isotopes that make up the element. 2. multiply the decimal fraction by the mass of the isotope to obtain the isotope contribution to the atomic mass. 3. sum these partial weights to get the weighted avg. atomic mass of element. Dalton"s atomic theory= the first experimentally based theory of atomic structure of the atom. True: all matter consists of tiny particles called atoms. False due to radioactivity: an atom cannot be created, divided, destroyed, or converted to any other type of atom.
Get access
Grade+
$40 USD/m
Billed monthly

Homework Help
Study Guides
Textbook Solutions
Class Notes
Textbook Notes
Booster Class
10 Verified Answers
Class+
$30 USD/m
Billed monthly

Homework Help
Study Guides
Textbook Solutions
Class Notes
Textbook Notes
Booster Class
7 Verified Answers
Related textbook solutions
Chemistry: Structure and Properties
2 Edition,
Tro
ISBN: 9780134293936
Basic Chemistry
5 Edition,
Timberlake
ISBN: 9780134138046
Principles of Chemistry Molecular Approach
4th Edition,
Tro
ISBN: 9780134112831
Chemistry: Structure and Properties
2nd Edition,
Tro
ISBN: 9780134293936
Principles of Chemistry Molecular Approach
3rd Edition, 2014
Tro
ISBN: 9780321971944
Chemistry: A Molecular Approach
3rd Edition,
Tro
ISBN: 9780321809247
Chemistry: A Molecular Approach
5th Edition,
Tro
ISBN: 9780134874371
Principles of Chemistry: A Molecular Approach
4th Edition,
Tro
ISBN: 9780134895741
Chemistry: The Central Science
14th Edition, 2017
Brown
ISBN: 9780134414232