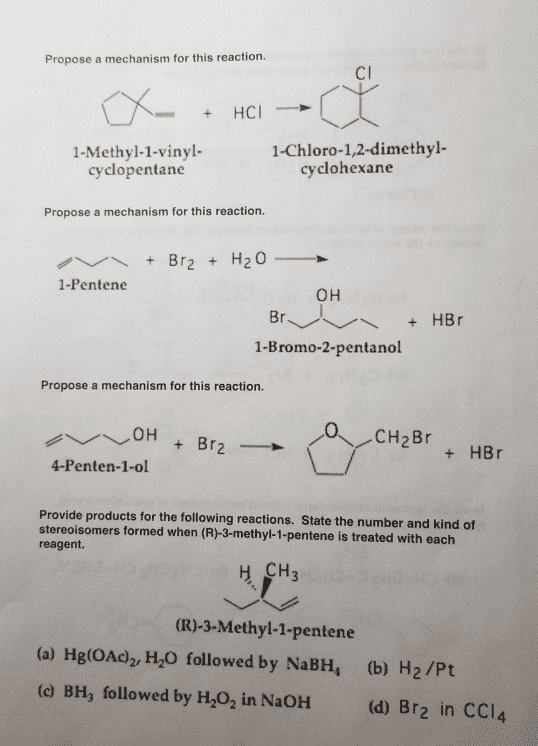
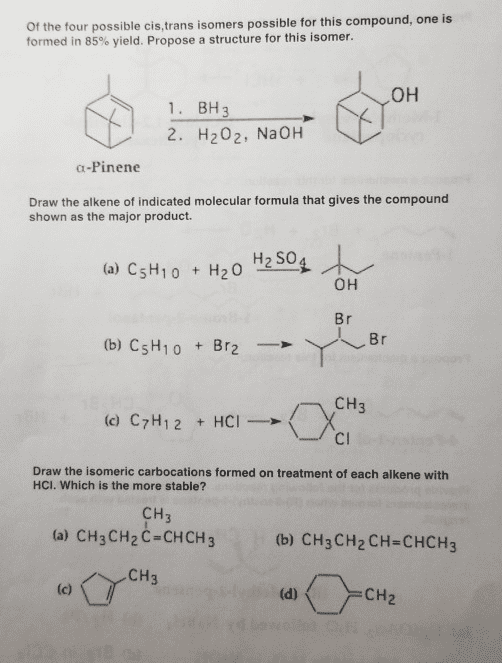
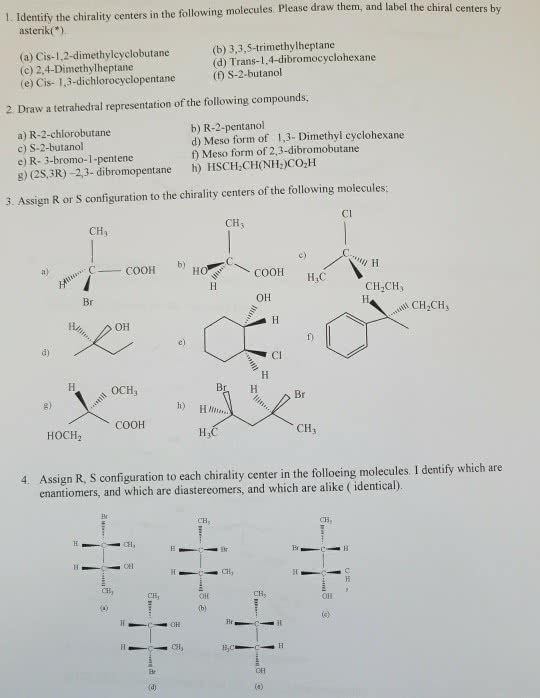
CHEM 130 Lecture Notes - Lecture 5: Pi Bond, Stereoisomerism, Substituent
Document Summary
The carbon-carbon double bond is formed between two sp2 hybridized carbons, and consists of two occupied molecular orbitals, a sigma orbital and a pi orbital. Rotation of the end groups of a double bond relative to each other destroys the p-orbital overlap that creates the pi orbital or bond. Because the pi bond has a bond energy of roughly 60 kcal/mole, this resistance to rotation stabilizes the planar configuration of this functional group. As a result, certain disubstituted alkenes may exist as a pair of configurational stereoisomers, often designated cis and trans. The essential requirement for this stereoisomerism is that each carbon of the double bond must have two different substituent groups (one may be hydrogen). This is illustrated by the following general formulas. In the first example, the left-hand double bond carbon has two identical substituents (a) so stereoisomerism about the double bond is not possible (reversing substituents on the right-hand carbon gives the same configuration).