Part 1 Boyle’s Law P1 V1 = P2 V2 when Temperature is constant; being 1 “initial” and 2 “final” A container of oxygen gas has a volume of 150 mL when its pressure is 0.947 atm. If the temperature remains constant. What will the volume of the gas be at a pressure of 0.987 atm? Using Boyle’s Law solve for V2 of final volume using P1 V1 = P2 V2 Match the correspondent given data, unknown data and resulted in data after solved
___1. Initial Volume or V1
___2. Final Pressure or P2
___3. Initial pressure or P1
___4. Final V2
___5 Temperature
A. does not change, remains constant
B. 144 mL of oxygen
C. 0.947 atm
D. 150 mL of oxygen
E. 130 mL of oxygen
F. 0.987 atm
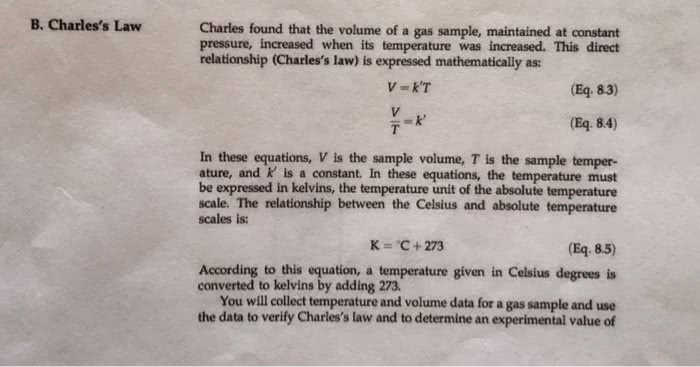
Part 2 Charles’s Law V1/T1=V2/T2 when the Pressure is constant; being 1 “initial” and 2 “final A container with hydrogen gas has a volume of 349 mL, at a temperature of 22.0C (Change to K), What volume will the gas occupy at 50C(Change to K), if the pressure remains constant?
____6. Final Volume of gas
____7 Initial Temperature gas
____8. Final Temperature gas
____9. Initial Volume gas
____10. The pressure of hydrogen
G. It’s constant
H. 295 K
I. 349 mL
J. 382 mL
K. 323 K
L. 50 C
Part 3 Gay-Lussac Law P1/T1=P2 when the volume (V) is constant A cylinder of a gas has a pressure of 4.40 atm at 25C (Change to K). At what temperature in Celsius will it reach a pressure of 6.50 atm C= K-273
____11. Final Pressure of gas (P1)
____12. The initial temperature in K (T1)
____13. The initial Pressure of gas (P2)
____14. The volume of the gas
_____15. The final temperature of the gas
M. 6.50 atm
N. 298 K
O. 4.40 atm
P. 167 C
Q . 283 K
R. is constant
Part 4 Combined Gas Law P1 V1/ T1 = P2 V2/ T2 OR MORE EASY TO SOLVE P1 V1 T2= P2 V2 T1 A St. Valentine Helium-filled balloon for your love, has a volume of 50.0 mL at 25 C (change to K) and 1.08 atm. What volume(V2) will it have at 0.855 atm and 10.0C (change to K). K=C + 273
_____16. The Final Temperature in K (T1)
_____17. The initial Volume in mL (V1)
_____18. The final Pressure in atm (P2)
_____19. The initial temperature in C (T1)
_____20. The final volume (V2)
S. 298k
T. 50.0 mL
U. 0.855 atm
V. 25 C
W. 283 K
X. 60.00 mL
Part 5 Answer with the letter in the blank. A. Boyles’s Law B. Charles’s Law C. Gay-Lussac’s Law D. Combined Gas Law E. Avogadro’s Law
___21. One mol of any gas at Standard Temperature and Pressure contains 6.03 X10^23 molecules and occupy a volume of 22.4L
____22. The higher the pressure the lower the volume when the temperature is constant.
____23. The higher the temperature the higher the volume when the pressure is constant.
____24 The higher the temperature the higher is the pressure when the volume is constant.
____25. There is a relation between pressure, volume, and temperature of a fixed amount (moles)of gas