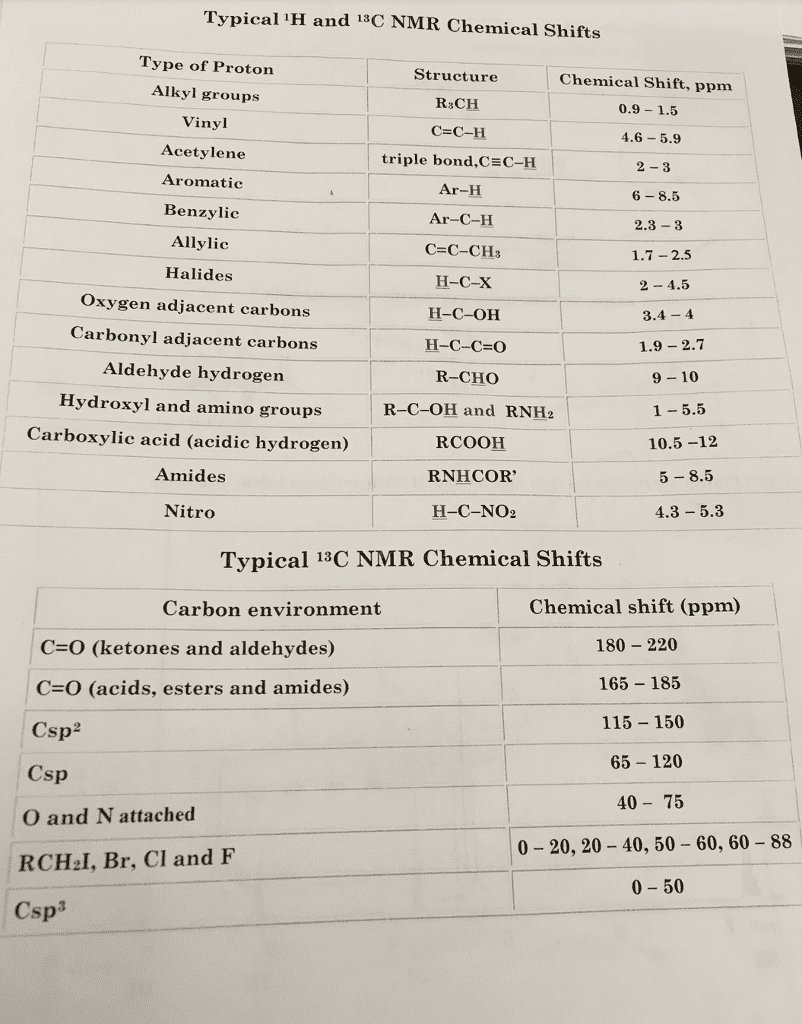
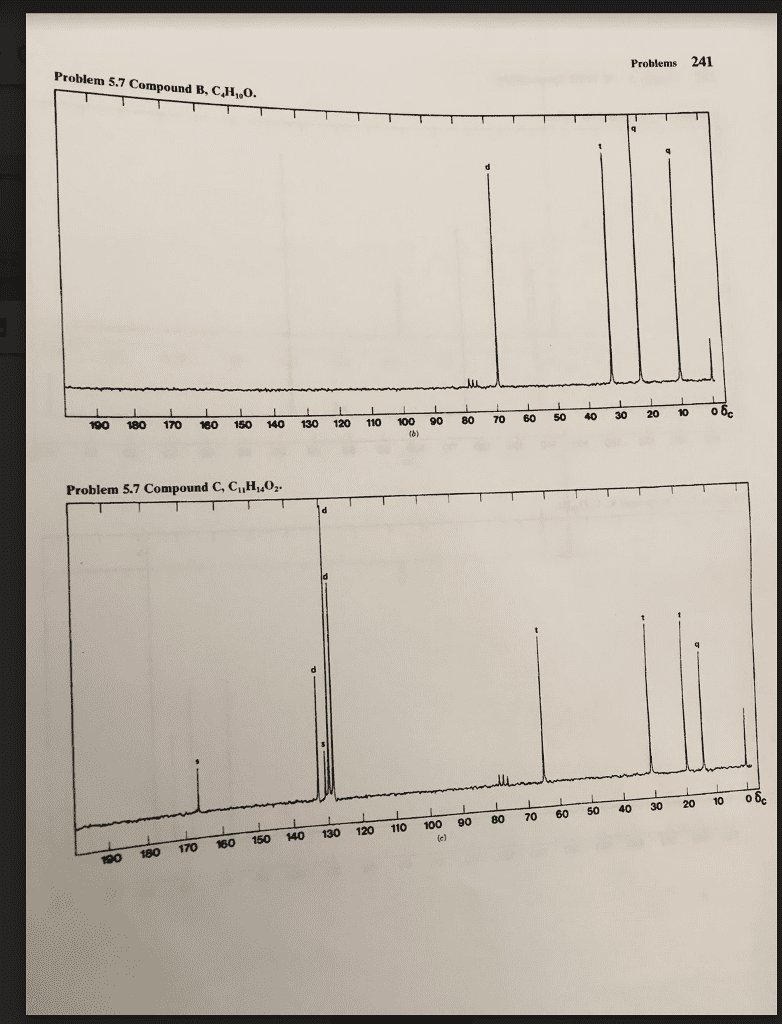
Test 1-Multiple Choice: Choose and underline the best answer in each of the following statement/question.
_____1. Which of the following best differentiates an aldehyde from a ketone?
a. In aldehydes, carbon is bonded to a hydroxyl group.
b. In aldehydes, hydrogen is bonded to an oxygen group.
c. In aldehydes, hydrogen is bonded to a carbonyl group.
d. In aldehydes, oxygen is bonded to an oxygen group.
_____2. Which of statement is not true about petroleum and its products?
a. Petroleum is fossil fuel.
b. Petroleum is a mixture of hydrocarbons with sulfur as impurity.
c. There is no harm that comes from the use of petroleum products.
d. Petroleum comes from decomposition of materials of living origin.
_____3. Cooking gas or liquefied petroleum gas(LPG) is a mixture of C3H8 and C4H10. Which are these compounds?
a. pentane and butane b. methane and butane
c. propane and butane d. propane and methane
_____4. Which of the following compounds ionizes in water and, thus conducts electricity poorly?
a. acetic acid b. hydrochloric acid c. distilled water d. sodium hydroxide
_____5. A lead storage cell can function both as an electrolytic cell and as a voltaic cell. When a lead storage battery is being recharged, it converts ________ energy to_____--energy
a. chemical : electrical b. chemical to heat c. electrical to mechanical
_____6. The acid electrolyte in a lead storage battery is
a. HCl b. H2SO4 c. HNO3 d. CH3COOH
____7. The anode and cathode in a dry cell respectively are
a. Zn and C b. C and Zn c. Pb and PbO2 d. PbO2 and Pb
____8. What is the proper name for the compound CH3CH2CH3?
a. ethane b. propane c.butane d. pentane
____9. Which of the following is the simplest hydrocarbon?
a. CH2 b. C2H4 c. CH3 d. CH4
____10. Hydrocarbons such as CH2 =CH2 are said t be
a. ionic b. aromatic c. saturated d. unsaturated
_____11. Which of the following best describes hydrocarbons?
a. flammable ionic compounds that are insoluble in water and are denser than water
b. flammable ionic compounds that are insoluble in water and are denser than water
c. flammable covalent compound that are insoluble in water and are less dense than water
d. flammable covalent compounds that are soluble in water and less dense than water
_____12. Which of the following will result if hexane and water are mixed?
a. an explosion
b. clear solution of hexane dissolved in water
c. a layer of hexane on top of a layer of water
d. a layer of water on top of a layer of hexane
_____13.Which of the following is not a gas?
a. ethene b. octane c. methane d. acetylene
_____14. Which of the following is another name for wood alcohol?
a. methanol b. propanol c. grain alcohol d. rubbing alcohol
_____15. Which of the following alcohols groups are found in beverages?
a. ethyl b. methyl c. denatured d. wood
_____16. Which of the following is the only element present in organic compounds?
a. hydrogen b. oxygen c. carbon d. nitrogen
_____17. Which of the following is chlorofluorocarbon?
a. C3F8 b. CHF3 c. CFCl3 d. CHCl3
_____18.What percent of alcohol is present in vodka which is 90 proof?
a. 9 b. 45 c. 90 d. 180
____20. Which of the following is formed when carbohydrates undergo fermentation process?
a. ethanol b. methanol c. glucose d. formaldehyde
_____21. In ethene, the two carbon atoms are joined by a
a.single bond b. double bond c. triple d. ionic
____22. The IUPAC name of CH3CH2CHCH2CH3 is
â
CH2CH3
a. 3-methylpentane b. 3-ethylpentane c. 2-ehtylpentane d. 2-methylpentane
____23. The IUPAC name of CH3-O-CH3 is
a. methoxymethane b. ethylether c. dimethylether d. dimethyloxide
____24. Which is an alcohol?
a. C6H6
b. CH3-C-CH3
â
O
c. CH3-O-CH3
d. CH3OH
____25. A group which both aldehydes and ketones have in common is
a.-C=O b. âOH c. âO- d. -COOH
___26. The aroma associated with this class of compounds is regarded as pleasant.
a. amides b. esters c. carboxylic acids d. ketones
____27. The IUPAC name of CH3CH2CHO is
a. propanone b. propanol c. propanal d. propionic acid
____28.The âCOOH group is called
a. aldehyde group b. hydroxyl group c. carbonyl group d. carboxylic group
____29.The alkyl group of butane is
a. C2H3 b. C3H5 c.C4H7 d. C4H9
____30. The simplest alkene is
a. CH=CH b. CH2=CH3 c. CH2=CH2 d. CH3CH=CH2
Test 2. Answer the following questions completely.(3 points each)
1.Why are alcohols of lower molecular weight more soluble in water than those with higher molecular weights?
2. Which of the following pairs of compounds has
a. higher bolilng point:1-bromopentane or 1-chloropentane _____________________
b. the greater density: heptanes or octane ______________________
c. the higher boiling point :pentyl alcohol or pentylamine ______________________
d. the higher boiling point: hexylamine or dipropylamine ______________________
3. Explain why each of the following names are incorrect.Give the correct IUPAC name.
a. 3-Butanone
b. 1-Butanone
4. What is unique about carbon atom that makes it form numerous compounds?
5. Acetone or propanone is completely soluble in water,but 4-heptanone is completely insoluble.Explain.
Test 3.. Give the name and chemical formula of the functional group of each of the following organic groups. The first one has been done for you.