CHMB41H3 Lecture 1: CHMB41 - Chapter 1
Document Summary
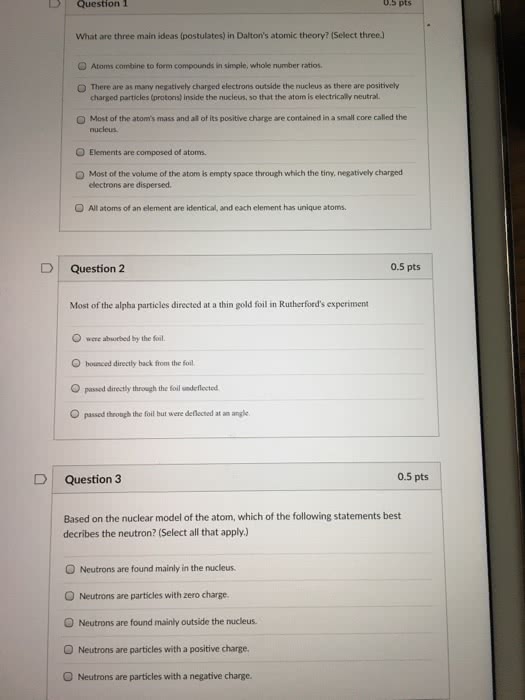
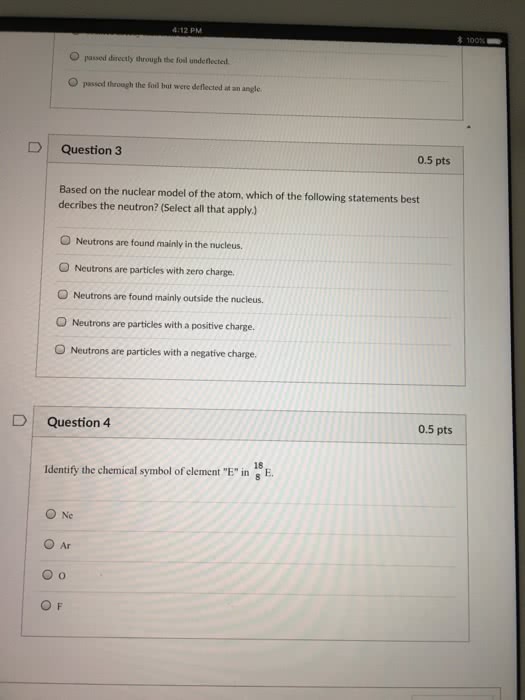
Chapter 1 remembering general chemistry: electronic structure & bonding. Jons jakob berzelius: compounds derived from living organisms organic, compounds derived from minerals inorganic. Organic compounds are compounds that contain carbon. Carbon neither readily gives up nor accepts e-, it shares e- Bonds form when two atoms share e- and bonds break when two atoms no longer share e- The nucleus contains positively charged protons & uncharged neutrons, so it is positive. Most of the mass of an atom is in its nucleus. Most of the volume is occupied by e- The atomic # is the number of protons in the nucleus. The mass # is the sum of protons and neutrons. The atomic weight is the avg mass of the atoms in the element. 1. 2 how the electrons in an atom are distributed. Louis de broglie e- have wavelike properties.