NUTR 3210 Lecture Notes - Lecture 7: Branched-Chain Amino Acid, Protein Quaternary Structure, Aminopeptidase

NUTR3210 – Proteins
What is Protein?
• Macronutrient
• **Health Canada recommends that protein intake comprises 10-30% of total energy
(ME)
• Main Functions:
o Protein synthesis
o Support of blood glucose
o Energy, ME=4 kcal/g
• **Requirements are for amino acids rather than whole proteins
• Very important: National and international recommendations for protein intake are based
on animal sources of protein (such as meat, milk and eggs)
o Plant proteins may be less digestible due to intrinsic differences in the nature of
the protein and the presence of other factors (like fibre which reduces protein
digestibility by <10%)
• Protein requirements vary with life stage (e.g. infancy, childhood, adolescence,
pregnancy)
Amino Acid Structure:
• *see slide
• connected by peptide bonds:
o amino acids join through condensation reactions, in which water is lost by a
reaction between the carboxyl group of one amino acid + amino group of another
o peptide bonds eliminate charge and decrease water solubility zwitterionic free
amino acids have a high water solubility
Protein Synthesis:
• peptide cannot be used interchangeable with protein quaternary structure
o 2 aa = dipeptide
o 3 aa = tripeptide
o 4-50 aa = olgiopeptide
o >50 aa = polypeptide
o *aa are added one at a time in the correct sequence
o 1 or more polypeptides biologically active protein
• linear polypeptide chain 3D folded protein (tertiary/quaternary structure)
Native vs Denatured
• native protein normal 3D configuration
• proteins are denatured by:
o heat
o salt treatment
o detergents
find more resources at oneclass.com
find more resources at oneclass.com

o pH (acids; stomach acid with HCl)
• When denatured, protein loses biological activity
• Denaturation affects secondary-quaternary structure (peptide bonds between aa are intact)
• Result: open up the protein so enzymes break peptide bonds
• E.g. Albumin (egg protein)
o Albumin in native form is transparent and liquid
o When cooked, the albumin becomes opaque and hard denatured
Amino Acid Classification
• Essential 9 in adults; 10 in infants *cannot be made by the body or made quickly
o Lysine
o Threonine
o Isoleucine*
o Leucine*
o Methionine
o Phenylalanine
o Tryptophan
o Valine*
o Histidine
o Arginine (in infants)
o * = branched chain amino acids
• Non-essential
o Can be synthesized in the body and are not essential components of the diet
Protein Digestion:
• Dietary protein homogenization (mouth: mechanical, lubrication)
• protein denaturation in the stomach (HCl)
• denatured protein olgiopeptides (by zymogens activating enzymes; activator=HCl)
o pepsinogen pepsin
o parapepsinogen 1&2 parapepsin 1&2
o **process occurs by proteolytic cleavage or HCl activation*
• olgiopeptide (from stomach) small intestine
o s. intestine secretes aminopeptidase (already active; not a zymgogen) and
enterokinase (activates trypsin)
o pancreas secretes trypsinogen, chymotyrpsinogen, proelastase,
procarboxypeptidase A&B
▪ *trypsin is activated first then trypsin activates other proteolytic enzymes
• free amino acids and small peptides
Enzymes:
• exopeptidases:
o amino peptidase cleaves off NH2 from amino acids
o carboxypeptidases cleaves COOH from end
• endopeptidases:
o trypsin cleaves basic aa peptide bonds inside amino acid chain
o elastase cleaves neutral aa peptide bonds
find more resources at oneclass.com
find more resources at oneclass.com
o pepsin, parapepsins, chymotrypsin cleaves large neutral aa peptide bonds (with
complex side chains)
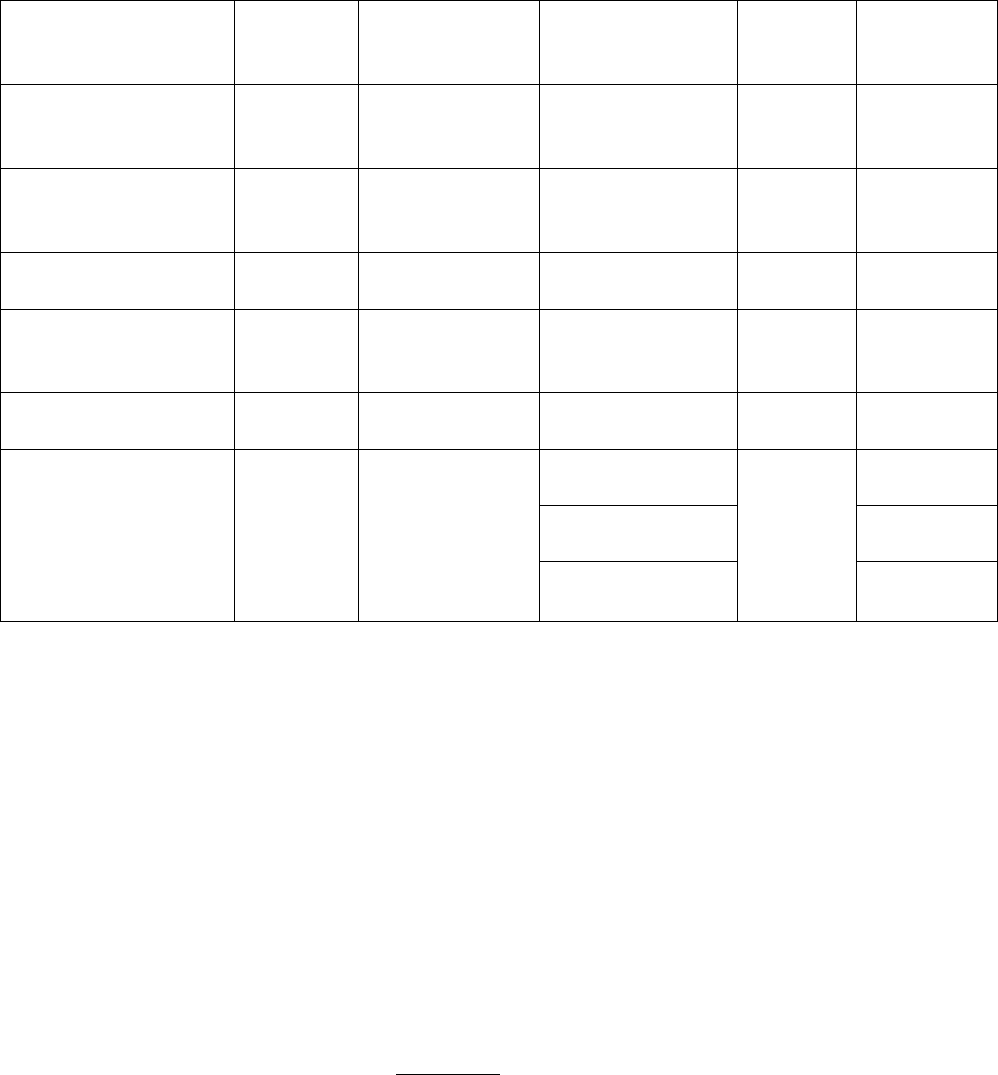
*See Secretion Summary:
• enterokinase (enteropeptidase) and aminopeptidase are secreted by the intestinal mucosa
in active forms NOT a zymogen
Zymogen (inactive)
Source of
Zymogen
Secretion
Enzyme/Activa
tor
Enzyme (active)
Site of
Activity
Substrate
Pepsinogen
Stomach
HCl or pepsin
Pepsin
Stomach
Most amino
acids (no
terminal aa)
Parapepsinogen 1&2
Stomach
HCl
Parapepsin 1&2
Stomach
Most amino
acids (no
terminal aa)
Trypsinogen
Pancreas
Enteropeptidase
Trypsin
S.
Intestine
Basic aa
Chymotrypsinogen
Pancreas
Trypsin
Chymotrypsin
S.
Intestine
Aromatic
aa, Met,
Asn, His
Proelastase
Pancreas
Trypsin
Elastase
S.
Intestine
Neutral aa
Procarboxypeptidase
A&B
Pancreas
Trypsin
Carboxypeptidase
A
Small
Intestine
C-terminal
neutral aa
Carboxypeptidase
B
C-terminal
basic aa
Aminopeptidases
N-terminal
aa
Amino Acid Absorption into Enterocytes (through Apical Membrane)
1. Facilitated Diffusion
• Depends on concentration gradient; high [aa] in the lumen and low [aa] inside
• Diffusion cannot occur if intracellular concentration of aa rises above
concentration in the lumen
• Does not require Na+ or ATP
2. Sodium-dependent amino acid transporters (active transport)
• Aa and Na+ are cotransported (Na+ moves down concentration gradient)
• Requires ATP to maintain Na concentration gradient
• Di/Tri-peptides (small) are cotransported by PEPT1
▪ Once inside the ell, they are further broken down by cytosolic peptidase
into individual amino acid
Protein Absorption: Enterocyte Circulation
• Amino acids cross the enterocyte basolateral membrane by facilitated diffusion
find more resources at oneclass.com
find more resources at oneclass.com
Document Summary
Albumin (egg protein: albumin in native form is transparent and liquid, when cooked, the albumin becomes opaque and hard denatured. *see secretion summary: enterokinase (enteropeptidase) and aminopeptidase are secreted by the intestinal mucosa. Substrate in active forms not a zymogen. Protein absorption: enterocyte circulation: amino acids cross the enterocyte basolateral membrane by facilitated diffusion. In wheat, lysine is the limiting amino acid (has lowest cs) Inadequate intake of protein (or intake of low quality protein) will cause n balance to shift negatively fail to sustain growth and maintenance of body protein. *see use of amino acids in anabolism: peptide hormones, transport/carrier proteins (albumin, regulatory proteins, structural proteins, transporters/receptors and enzymes. Nutritionally relevant protein modification **return in vitamins and minerals section. Post translational modifications occur at different amino acid residues within protein. Phosphorylation: addition of pi to a protein; requires micronutrient phosphorus (widely distributed in both plants and animal foods)

