BCH 261 Lecture 12: Biochem Notes 12
Document Summary
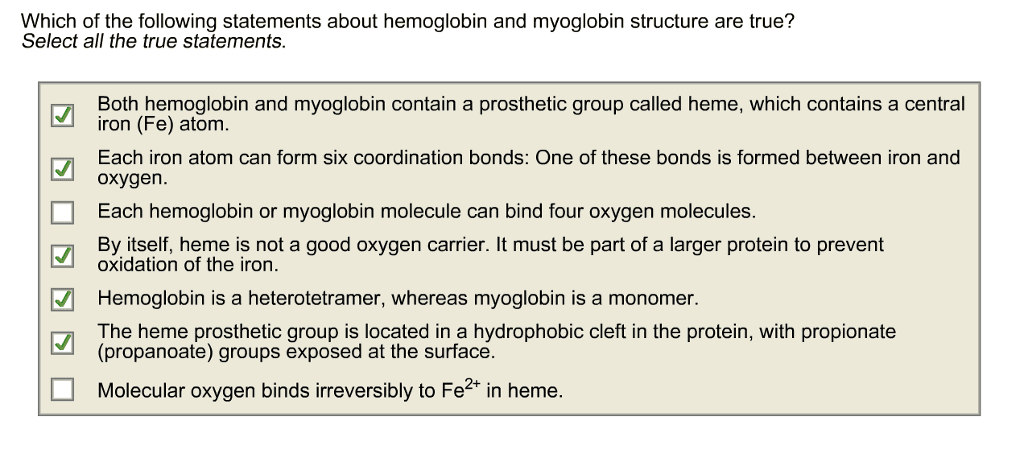
To prevent free radicals fe+2 is coupled to an organic group called. Fe2+ (can bind o2) in free heme can be oxidized to fe3+ (cannot bind o2). Sequester the coupled heme-o2 molecules within the proper protein milieu: myoglobin. In mammals, myoglobin is the main oxygen storage protein structure of heme. Heme is a protoporphyrin - porphyrins have four pyrrole rings (circled) Fe+2 has six coordination bonds, four are coordinated with four n, and two are perpendicular to the ring"s plane. Heme secured by a hydrophobic pocket that prevents heme from leaving. Carbon monoxide kills because it binds heme better than o2. Co is highly toxic as it competes with oxygen. It blocks the function of myoglobin, hemoglobin, and mitochondrial cytochromes. Co binds to free heme > 20,000 times better than o2. Is a tetramer (complex of 4 proteins, each with a heme group) Binds o2 in lungs and delivers to muscle and other cells.