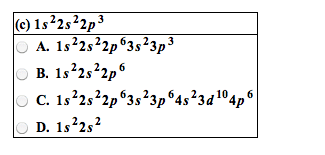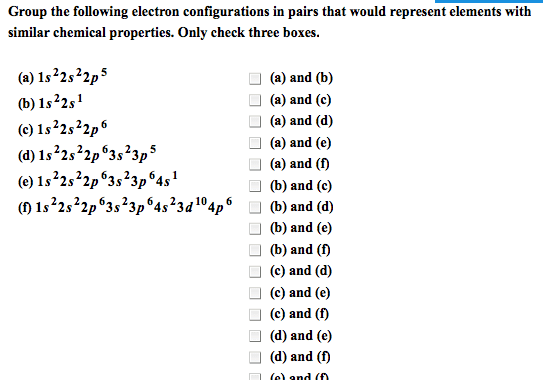CHEM 208 Lecture Notes - Lecture 3: Ionic Compound, Nonmetal, Atomic Number
Document Summary
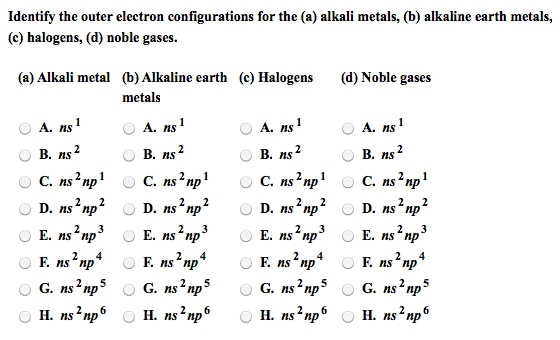
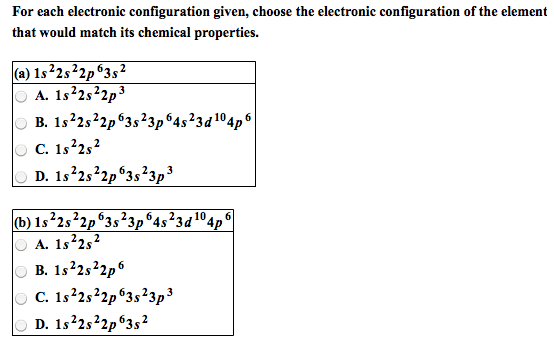
Module 3: the periodic table and naming compounds. The periodic table is arranged by increasing atomic number of the element (which is equal to the number of protons in the nucleus of its atoms) Vertical columns called groups (families) and horizontal rows called periods (numbered 1-7) Elements in the same group have similar chemical properties. Elements in a period have different chemical properties. Designated with the numbers 1-8 (i-viii) or a or b. Inner transition elements (lanthanides and actinides) are located between 3b-4b. Group 8a: noble gases (rare or inert) Organic: contain at least one carbon atom. Inorganic: contains atoms other than carbon, exception (co) (co2) Formed by electrostatic attraction between positive ion (cation from metal) and negative ion (anion from nonmetal) Electrons are transferred from the atoms of one element to another; metals lose electrons and nonmetals gain electrons. Ionic compounds are charge neutral (zero net charge)