30
answers
0
watching
204
views
1 Jun 2023
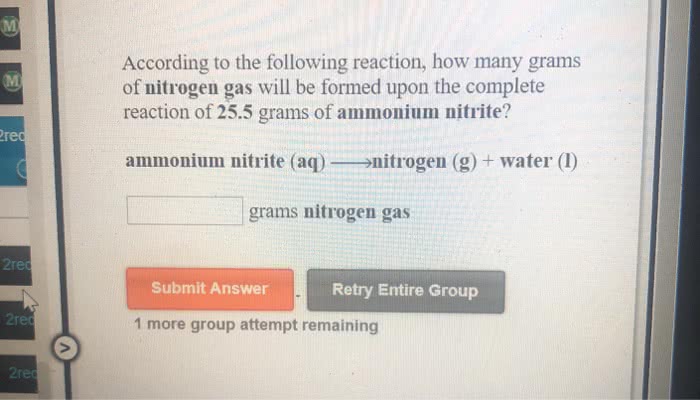
Ammonium nitrite decomposes to give off nitrogen gas and liquid water. How many grams of ammonium nitrite must have reacted if 2.58 L of gas was collected over water in a gas collecting tube at 21.0°C and 97.8 kPa?
Balanced equation:
Will the volume of nitrogen (from the previous problem) INCREASE, DECREASE or remain the SAME if... *Explain briefly*
...the experiment is done at significantly higher temperature?
B. ...the amount of ammonium nitrite was increased?
C.
...the experiment was not collected over water?
Ammonium nitrite decomposes to give off nitrogen gas and liquid water. How many grams of ammonium nitrite must have reacted if 2.58 L of gas was collected over water in a gas collecting tube at 21.0°C and 97.8 kPa?
Balanced equation:
Will the volume of nitrogen (from the previous problem) INCREASE, DECREASE or remain the SAME if... *Explain briefly*
...the experiment is done at significantly higher temperature?
B. ...the amount of ammonium nitrite was increased?
C.
...the experiment was not collected over water?
karimbalticLv7
3 Nov 2023
wahabmunir796Lv10
11 Jul 2023
Already have an account? Log in
3 Jun 2023
Already have an account? Log in
qamarmalik3098Lv10
3 Jun 2023
Already have an account? Log in
raj21chaharLv10
3 Jun 2023
Already have an account? Log in
2 Jun 2023
Already have an account? Log in
2 Jun 2023
Already have an account? Log in
2 Jun 2023
Already have an account? Log in
1 Jun 2023
Already have an account? Log in