3
answers
0
watching
17
views
12 Dec 2019
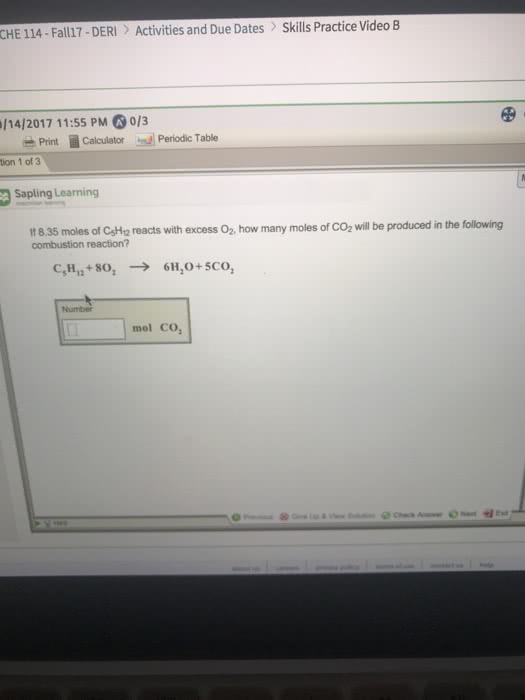
1)If 4.09 moles of C5H12 reacts with excess O2, how many moles of CO2 will be produced in the following combustion reaction?
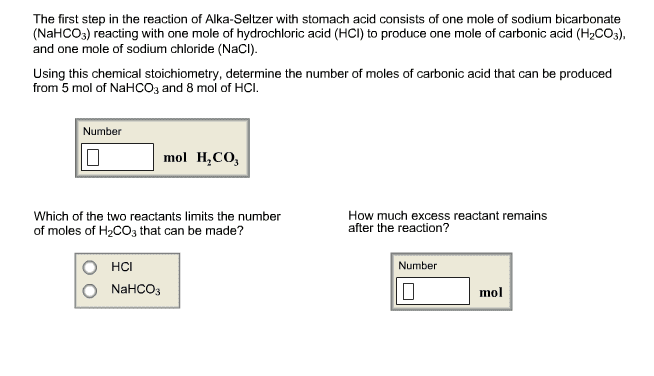
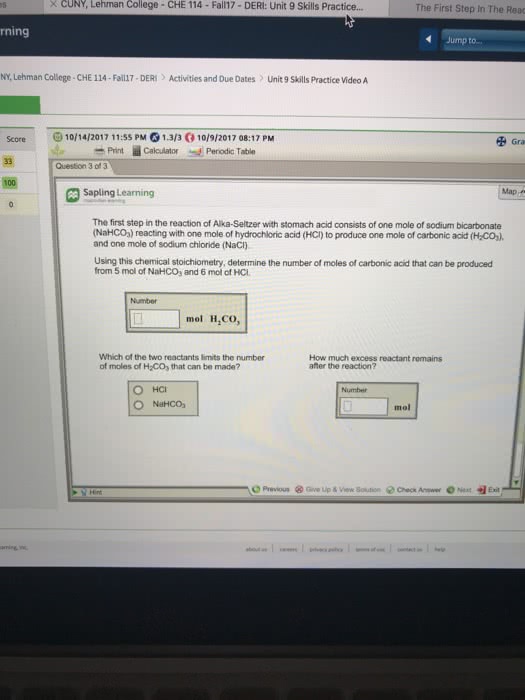
2)The first step in the reaction of Alka-Seltzer with stomach acid consists of one mole of sodium bicarbonate (NaHCO3) reacting with one mole of hydrochloric acid (HCl) to produce one mole of carbonic acid (H2CO3), and one mole of sodium chloride (NaCl).
Using this chemical stoichiometry, determine the number of moles of carbonic acid that can be produced from 5 mol of NaHCO3 and 8 mol of HCl.
How much excess reactant remains after the reaction?
1)If 4.09 moles of C5H12 reacts with excess O2, how many moles of CO2 will be produced in the following combustion reaction?
2)The first step in the reaction of Alka-Seltzer with stomach acid consists of one mole of sodium bicarbonate (NaHCO3) reacting with one mole of hydrochloric acid (HCl) to produce one mole of carbonic acid (H2CO3), and one mole of sodium chloride (NaCl).
Using this chemical stoichiometry, determine the number of moles of carbonic acid that can be produced from 5 mol of NaHCO3 and 8 mol of HCl.
How much excess reactant remains after the reaction?
5 Jun 2023
Unlock all answers
Get 1 free homework help answer.
Already have an account? Log in
2 Jun 2023
Get unlimited access
Already have an account? Log in
Sixta KovacekLv2
13 Dec 2019
Get unlimited access
Already have an account? Log in