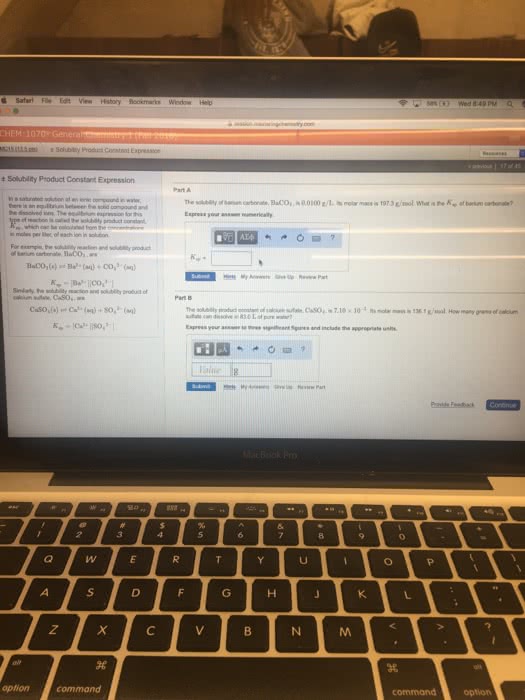
In a saturated solution of an ionic compound in water, there is an equilibrium between the solid compound and the dissolved ions. The equilibrium expression for this type of reaction is called the solubility product constant, Ksp, which can be calculated from the concentrations, in moles per liter, of each ion in solution.
For example, the solubility reaction and solubility product of barium carbonate, BaCO3, are
BaCO3(s)âBa2+(aq)+CO32â(aq)Ksp=[Ba2+][CO32â]
Similarly, the solubility reaction and solubility product of calcium sulfate, CaSO4, are
CaSO4(s)âCa2+(aq)+SO42â(aq)Ksp=[Ca2+][SO42â]
The solubility of barium carbonate, BaCO3, is 0.0100 g/L. Its molar mass is 197.3 g/mol. What is the Ksp of barium carbonate?â
The solubility product constant of calcium sulfate, CaSO4, is 7.10Ã10â5. Its molar mass is 136.1 g/mol. How many grams of calcium sulfate can dissolve in 57.0 L of pure water?â
In a saturated solution of an ionic compound in water, there is an equilibrium between the solid compound and the dissolved ions. The equilibrium expression for this type of reaction is called the solubility product constant, Ksp, which can be calculated from the concentrations, in moles per liter, of each ion in solution.
For example, the solubility reaction and solubility product of barium carbonate, BaCO3, are
BaCO3(s)âBa2+(aq)+CO32â(aq)Ksp=[Ba2+][CO32â]
Similarly, the solubility reaction and solubility product of calcium sulfate, CaSO4, are
CaSO4(s)âCa2+(aq)+SO42â(aq)Ksp=[Ca2+][SO42â]
The solubility of barium carbonate, BaCO3, is 0.0100 g/L. Its molar mass is 197.3 g/mol. What is the Ksp of barium carbonate?â
The solubility product constant of calcium sulfate, CaSO4, is 7.10Ã10â5. Its molar mass is 136.1 g/mol. How many grams of calcium sulfate can dissolve in 57.0 L of pure water?â