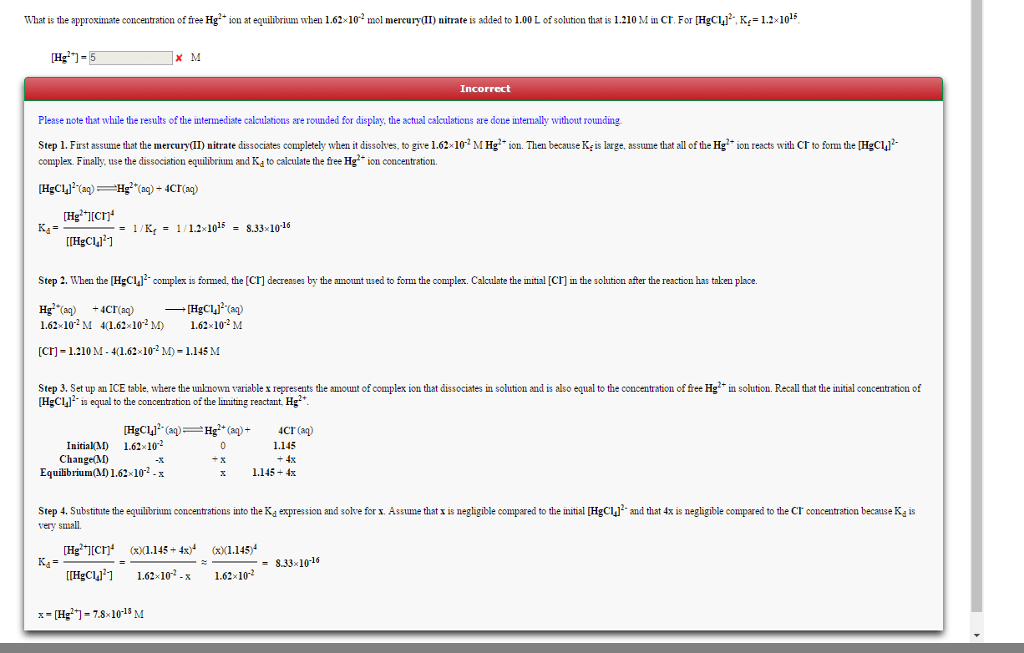
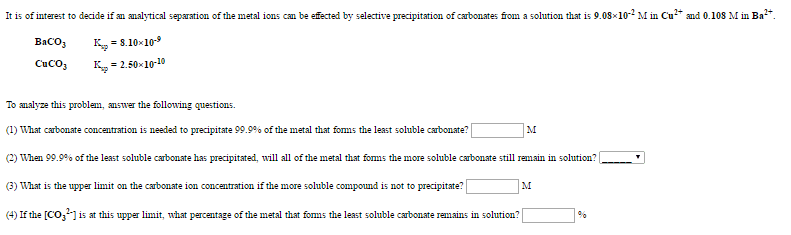
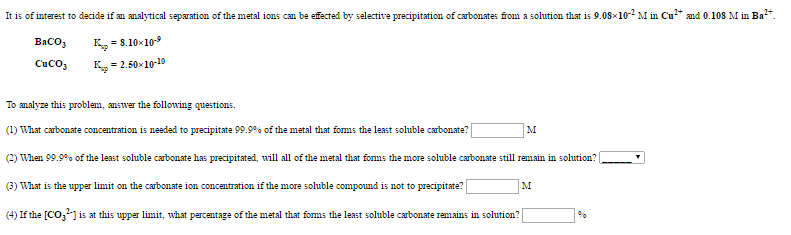
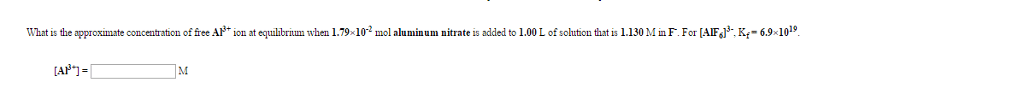An aqueous solution is prepared to be 0.256 M in sodium acetylsalicylate and 0.113 M in acetic acid.
(1) Is this solution a buffer solution? _____YesNo
(2) What is the pH of this solution? pH =
(3) If 0.122 moles of perchloric acid are added to one liter of this solution, what is the pH of the resulting solution? pH =
Use the Tables link in the References for any equilibrium constants that are required.
Acid/Base Ionization Constants at 25 oC
Acid
Formula
Ka1
Ka2
Ka3
Acetic acid
CH3COOH
1.8 Ã 10-5
Acetylsalicylic acid (aspirin)
HC9H7O4
3.0 Ã 10-4
Aluminum ion
Al(H2O)43+
1.2 Ã 10-5
Arsenic acid
H3AsO4
2.5 Ã 10-4
5.6 Ã 10-8
3.0 Ã 10-13
Ascorbic acid
H2C6H6O6
7.9 Ã 10-5
1.6 Ã 10-12
Benzoic acid
C6H5COOH
6.3 Ã 10-5
Carbonic acid
H2CO3
4.2 Ã 10-7
4.8 Ã 10-11
Ferric ion
Fe(H2O)63+
4.0 Ã 10-3
Formic acid
HCOOH
1.8 Ã 10-4
Hydrocyanic acid
HCN
4.0 Ã 10-10
Hydrofluoric acid
HF
7.2 Ã 10-4
Hydrogen peroxide
H2O2
2.4 Ã 10-12
Hydrosulfuric acid
H2S
1.0 Ã 10-7
1.0 Ã 10-19
Hypochlorous acid
HClO
3.5 Ã 10-8
Nitrous acid
HNO2
4.5 Ã 10-4
Oxalic acid
H2C2O4
5.9 Ã 10-2
6.4 Ã 10-5
Phenol
C6H5OH
1.0 Ã 10-10
Phosphoric acid
H3PO4
7.5 Ã 10-3
6.2 Ã 10-8
3.6 Ã 10-13
Sulfuric acid
H2SO4
very large
1.2 Ã 10-2
Sulfurous acid
H2SO3
1.7 Ã 10-2
6.4 Ã 10-8
Zinc ion
Zn(H2O)42+
2.5 Ã 10-10
Base
Formula
Kb
Ammonia
NH3
1.8 Ã 10-5
Aniline
C6H5NH2
7.4 Ã 10-10
Caffeine
C8H10N4O2
4.1 Ã 10-4
Codeine
C18H21O3N
8.9 Ã 10-7
Diethylamine
(C2H5)2NH
6.9 Ã 10-4
Dimethylamine
(CH3)2NH
5.9 Ã 10-4
Ethylamine
C2H5NH2
4.3 Ã 10-4
Hydroxylamine
NH2OH
9.1 Ã 10-9
Isoquinoline
C9H7N
2.5 Ã 10-9
Methylamine
CH3NH2
4.2 Ã 10-4
Morphine
C17H19O3N
7.4 Ã 10-7
Piperidine
C5H11N
1.3 Ã 10-3
Pyridine
C5H5N
1.5 Ã 10-9
Quinoline
C9H7N
6.3 Ã 10-10
Triethanolamine
C6H15O3N
5.8 Ã 10-7
Triethylamine
(C2H5)3N
5.2 Ã 10-4
Trimethylamine
(CH3)3N
6.3 Ã 10-5
Urea
N2H4CO
1.5 Ã 10-14
An aqueous solution is prepared to be 0.256 M in sodium acetylsalicylate and 0.113 M in acetic acid.
(1) Is this solution a buffer solution? _____YesNo
(2) What is the pH of this solution? pH =
(3) If 0.122 moles of perchloric acid are added to one liter of this solution, what is the pH of the resulting solution? pH =
Use the Tables link in the References for any equilibrium constants that are required.
| Acid/Base Ionization Constants at 25 oC | ||||
|---|---|---|---|---|
| Acid | Formula | Ka1 | Ka2 | Ka3 |
| Acetic acid | CH3COOH | 1.8 Ã 10-5 | ||
| Acetylsalicylic acid (aspirin) | HC9H7O4 | 3.0 Ã 10-4 | ||
| Aluminum ion | Al(H2O)43+ | 1.2 Ã 10-5 | ||
| Arsenic acid | H3AsO4 | 2.5 Ã 10-4 | 5.6 Ã 10-8 | 3.0 Ã 10-13 |
| Ascorbic acid | H2C6H6O6 | 7.9 Ã 10-5 | 1.6 Ã 10-12 | |
| Benzoic acid | C6H5COOH | 6.3 Ã 10-5 | ||
| Carbonic acid | H2CO3 | 4.2 Ã 10-7 | 4.8 Ã 10-11 | |
| Ferric ion | Fe(H2O)63+ | 4.0 Ã 10-3 | ||
| Formic acid | HCOOH | 1.8 Ã 10-4 | ||
| Hydrocyanic acid | HCN | 4.0 Ã 10-10 | ||
| Hydrofluoric acid | HF | 7.2 Ã 10-4 | ||
| Hydrogen peroxide | H2O2 | 2.4 Ã 10-12 | ||
| Hydrosulfuric acid | H2S | 1.0 Ã 10-7 | 1.0 Ã 10-19 | |
| Hypochlorous acid | HClO | 3.5 Ã 10-8 | ||
| Nitrous acid | HNO2 | 4.5 Ã 10-4 | ||
| Oxalic acid | H2C2O4 | 5.9 Ã 10-2 | 6.4 Ã 10-5 | |
| Phenol | C6H5OH | 1.0 Ã 10-10 | ||
| Phosphoric acid | H3PO4 | 7.5 Ã 10-3 | 6.2 Ã 10-8 | 3.6 Ã 10-13 |
| Sulfuric acid | H2SO4 | very large | 1.2 Ã 10-2 | |
| Sulfurous acid | H2SO3 | 1.7 Ã 10-2 | 6.4 Ã 10-8 | |
| Zinc ion | Zn(H2O)42+ | 2.5 Ã 10-10 | ||
| Base | Formula | Kb | ||
|---|---|---|---|---|
| Ammonia | NH3 | 1.8 Ã 10-5 | ||
| Aniline | C6H5NH2 | 7.4 Ã 10-10 | ||
| Caffeine | C8H10N4O2 | 4.1 Ã 10-4 | ||
| Codeine | C18H21O3N | 8.9 Ã 10-7 | ||
| Diethylamine | (C2H5)2NH | 6.9 Ã 10-4 | ||
| Dimethylamine | (CH3)2NH | 5.9 Ã 10-4 | ||
| Ethylamine | C2H5NH2 | 4.3 Ã 10-4 | ||
| Hydroxylamine | NH2OH | 9.1 Ã 10-9 | ||
| Isoquinoline | C9H7N | 2.5 Ã 10-9 | ||
| Methylamine | CH3NH2 | 4.2 Ã 10-4 | ||
| Morphine | C17H19O3N | 7.4 Ã 10-7 | ||
| Piperidine | C5H11N | 1.3 Ã 10-3 | ||
| Pyridine | C5H5N | 1.5 Ã 10-9 | ||
| Quinoline | C9H7N | 6.3 Ã 10-10 | ||
| Triethanolamine | C6H15O3N | 5.8 Ã 10-7 | ||
| Triethylamine | (C2H5)3N | 5.2 Ã 10-4 | ||
| Trimethylamine | (CH3)3N | 6.3 Ã 10-5 | ||
| Urea | N2H4CO | 1.5 Ã 10-14 |