1
answer
0
watching
168
views
6 Oct 2020
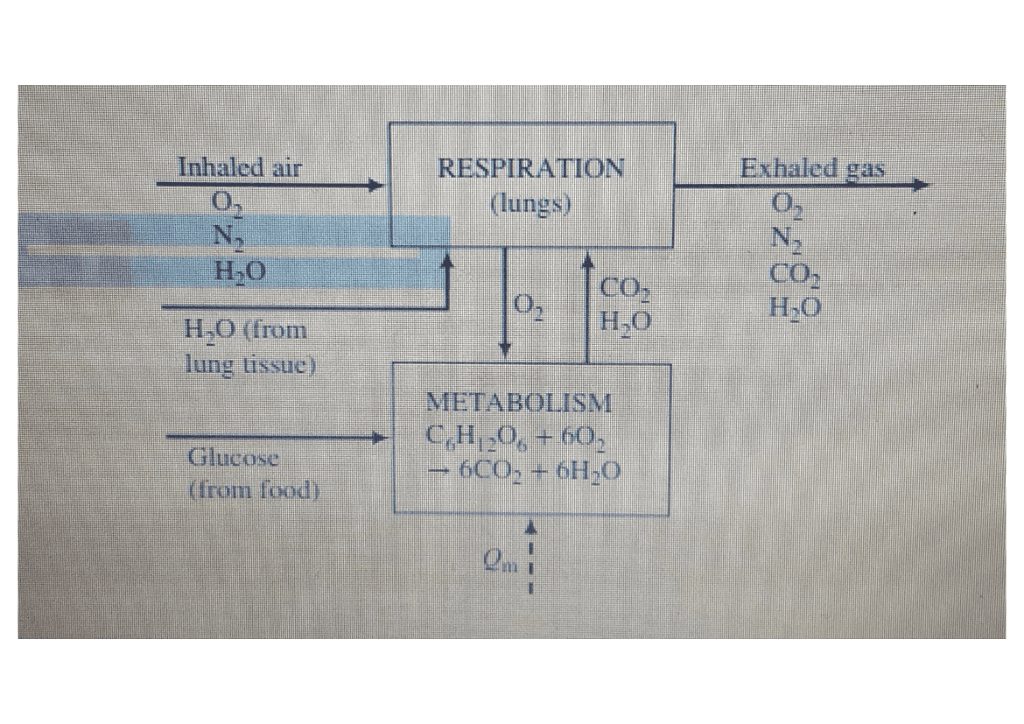
The excess internal energy of metabolism is exhausted through a variety of channels, such as through radiation and evaporation of perspiration. Consider another pathway for energy loss: moisture in exhaled breath. Suppose you breathe out 22.0 breaths per minute, each with a volume of 0.600 L. Suppose also that you inhale dry air and exhale at 37oC containing water vapor with a vapor pressure of 3.20 kPa. The vapor comes from the evaporation of liquid water in your body. Model the water vapor as an ideal gas. Assume its latent heat of evaporation at 37oC is the same as its heat of vaporization at 100oC. Calculate the rate at which you lose energy by exhaling humid air.
The excess internal energy of metabolism is exhausted through a variety of channels, such as through radiation and evaporation of perspiration. Consider another pathway for energy loss: moisture in exhaled breath. Suppose you breathe out 22.0 breaths per minute, each with a volume of 0.600 L. Suppose also that you inhale dry air and exhale at 37oC containing water vapor with a vapor pressure of 3.20 kPa. The vapor comes from the evaporation of liquid water in your body. Model the water vapor as an ideal gas. Assume its latent heat of evaporation at 37oC is the same as its heat of vaporization at 100oC. Calculate the rate at which you lose energy by exhaling humid air.
Bryllant BaluyutLv10
9 Nov 2020