1
answer
0
watching
691
views
6 Oct 2020
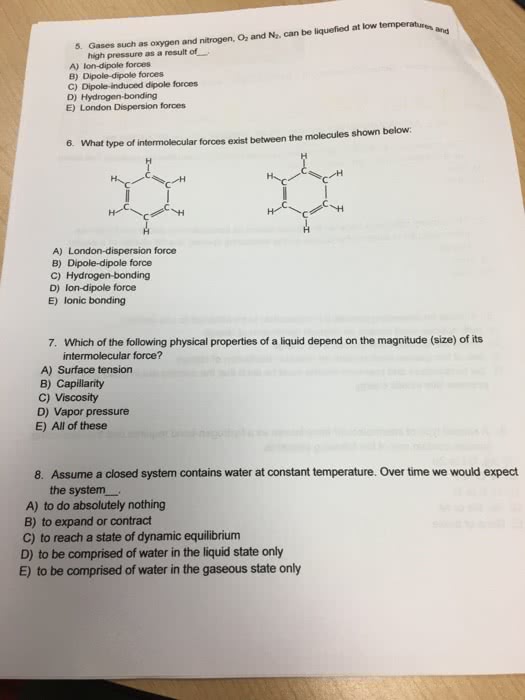
Which of the following statements about intermolecular forces is(are) true?
London dispersion forces are the only type of intermolecular force that nonpolar molecules exhibit.
Molecules that have only London dispersion forms will always be gases at room temperature (25°C).
The hydrogen-bonding forces in NH3 are stronger than those in H2O.
The molecules in SO2(g) exhibit dipole-dipole intermolecular interactions
CH3CH2CH3 has stronger London dispersion forces than does CH4.
Which of the following statements about intermolecular forces is(are) true?
London dispersion forces are the only type of intermolecular force that nonpolar molecules exhibit.
Molecules that have only London dispersion forms will always be gases at room temperature (25°C).
The hydrogen-bonding forces in NH3 are stronger than those in H2O.
The molecules in SO2(g) exhibit dipole-dipole intermolecular interactions
CH3CH2CH3 has stronger London dispersion forces than does CH4.
1
answer
0
watching
691
views
For unlimited access to Homework Help, a Homework+ subscription is required.
Christian TusoLv10
27 Nov 2020
Related textbook solutions
Basic Chemistry
5 Edition,
Timberlake
ISBN: 9780134138046
Principles of Chemistry Molecular Approach
4th Edition,
Tro
ISBN: 9780134112831
Chemistry: Structure and Properties
2nd Edition,
Tro
ISBN: 9780134293936
Principles of Chemistry Molecular Approach
3rd Edition, 2014
Tro
ISBN: 9780321971944
Chemistry: A Molecular Approach
3rd Edition,
Tro
ISBN: 9780321809247
Chemistry: A Molecular Approach
5th Edition,
Tro
ISBN: 9780134874371
Principles of Chemistry: A Molecular Approach
4th Edition,
Tro
ISBN: 9780134895741
Chemistry: The Central Science
14th Edition, 2017
Brown
ISBN: 9780134414232