1
answer
0
watching
225
views
15 Apr 2020
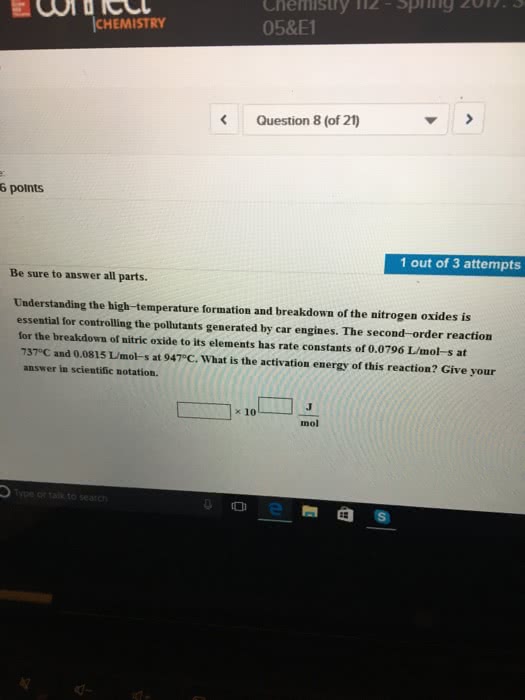
Understanding the high-temperature behavior of nitrogen oxides is essential for controlling pollution generated in automobile engines. The decomposition of nitric oxide (NO) to N2 and O2 is second order with a rate constant of 0.0796 M -1s-1 at 737 °C and 0.0815 M -1s-1 at 947 °C. Calculate the activation energy for the reaction.
Understanding the high-temperature behavior of nitrogen oxides is essential for controlling pollution generated in automobile engines. The decomposition of nitric oxide (NO) to N2 and O2 is second order with a rate constant of 0.0796 M -1s-1 at 737 °C and 0.0815 M -1s-1 at 947 °C. Calculate the activation energy for the reaction.
Deanna HettingerLv2
23 May 2020