1
answer
0
watching
307
views
11 Dec 2019
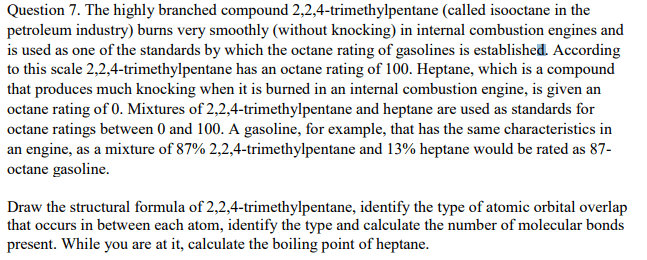
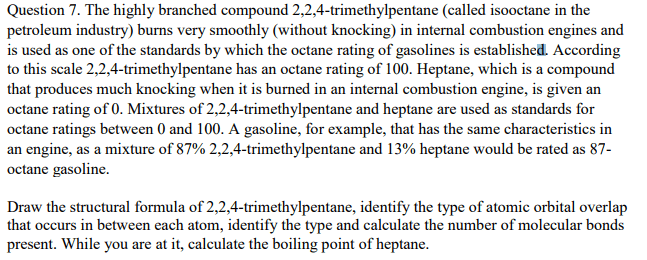
Isooctane, C8H18, is the basis of the octane rating system for gasoline because it burns smoothly, with minimal engine knocking. Pure isooctane is assigned a value of 100. Octane ratings are assigned to gasoline mixtures based on their ability to prevent knocking relative to isooctane. Estimate the energy released during the combustion of 1.00 mol of pure isooctane.
Isooctane, C8H18, is the basis of the octane rating system for gasoline because it burns smoothly, with minimal engine knocking. Pure isooctane is assigned a value of 100. Octane ratings are assigned to gasoline mixtures based on their ability to prevent knocking relative to isooctane. Estimate the energy released during the combustion of 1.00 mol of pure isooctane.
1
answer
0
watching
307
views
For unlimited access to Homework Help, a Homework+ subscription is required.
Hubert KochLv2
13 Dec 2019
Related textbook solutions
Basic Chemistry
5 Edition,
Timberlake
ISBN: 9780134138046
Principles of Chemistry Molecular Approach
4th Edition,
Tro
ISBN: 9780134112831
Principles of Chemistry Molecular Approach
3rd Edition, 2014
Tro
ISBN: 9780321971944
Chemistry: Structure and Properties
2nd Edition,
Tro
ISBN: 9780134293936
Chemistry: A Molecular Approach
3rd Edition,
Tro
ISBN: 9780321809247
Chemistry: A Molecular Approach
5th Edition,
Tro
ISBN: 9780134874371
Principles of Chemistry: A Molecular Approach
4th Edition,
Tro
ISBN: 9780134895741
Chemistry: The Central Science
14th Edition, 2017
Brown
ISBN: 9780134414232