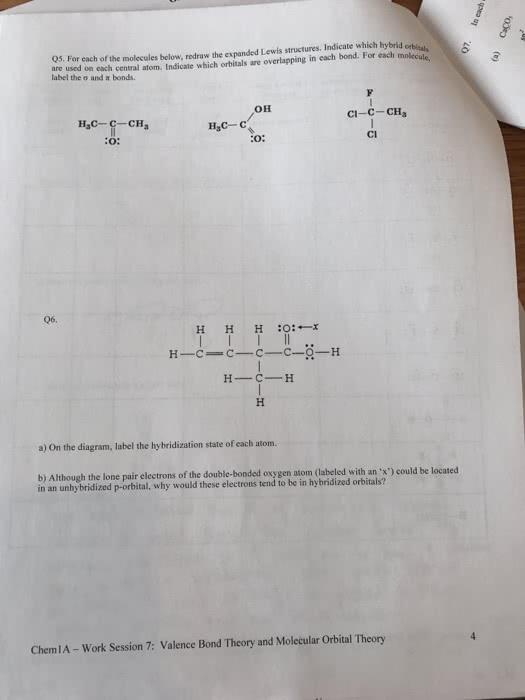
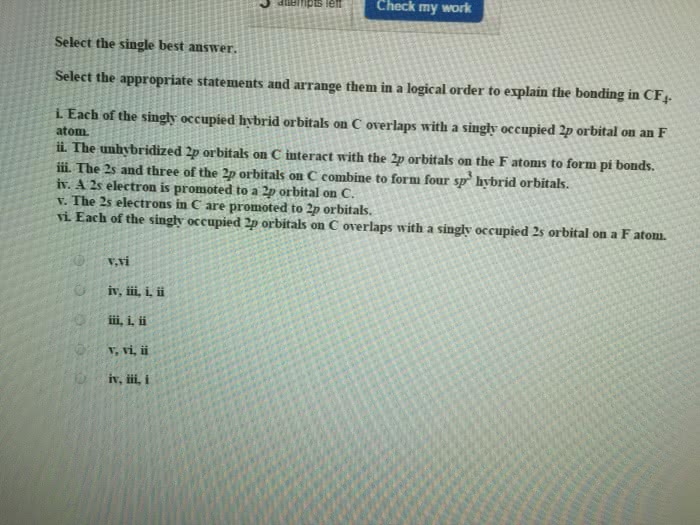
Use valence bond theory to describe the bonding and geometry in theSeF4 molecule.
A. The central atom is Se, which has five electron groups around it(four bonding pairs and one nonbonding/lone pair). Theelectron-group geometry is trigonal bipyramidal (and the moleculargeometry is see-saw). To facilitate this geometry, the Se atom'sorbitals are hybridized as sp3d orbitals. Four of these hybridorbitals contain one of Se's 6 valence electrons each, and thesehybrid orbitals overlap with unhybridized p orbitals of the four Fatoms. The fifth hybrid orbital of Se contains a lone pair ofelectrons. There are 4 sigma bonds and 0 pi bonds in thismolecule.
B. The central atom is Se, which has six electron groups around it.The electron-group geometry is octahedral. To facilitate thisgeometry, the Se atom's orbitals are hybridized as sp3d2 orbitals.Four of these hybrid orbitals contain one of Se's 6 valenceelectrons each, and these hybrid orbitals overlap with unhybridizedp orbitals of the four F atoms. The fifth and sixth hybrid orbitalsof Se contain lone pairs of electrons. There are 4 sigma bonds and0 pi bonds in this molecule.
C. The central atom is Se, which has four electron groups aroundit. The electron-group geometry is tetrahedral. To facilitate thisgeometry, the Se atom's orbitals are hybridized as sp3 orbitals.These hybrid orbitals overlap with unhybridized p orbitals of thefour F atoms. There are 4 sigma bonds and 0 pi bonds in thismolecule.
D. The central atom is Se, which has four electron groups aroundit, two single bonds and two double bonds. The electron-groupgeometry is tetrahedral. To facilitate this geometry, the Se atom'sorbitals are hybridized as sp3 orbitals. These hybrid orbitalscontain one electron each of Se's 6 valence electrons, and thesehybrid orbitals overlap with unhybridized p orbitals of the four Fatoms. The final 2 electrons of Se's 6 valence electrons reside inunhybridized p orbitals, which overlap with unhybridized p orbitalsof the F atoms. There are 4 sigma bonds and 2 pi bonds in thismolecule.
Use valence bond theory to describe the bonding and geometry in theSeF4 molecule.
A. The central atom is Se, which has five electron groups around it(four bonding pairs and one nonbonding/lone pair). Theelectron-group geometry is trigonal bipyramidal (and the moleculargeometry is see-saw). To facilitate this geometry, the Se atom'sorbitals are hybridized as sp3d orbitals. Four of these hybridorbitals contain one of Se's 6 valence electrons each, and thesehybrid orbitals overlap with unhybridized p orbitals of the four Fatoms. The fifth hybrid orbital of Se contains a lone pair ofelectrons. There are 4 sigma bonds and 0 pi bonds in thismolecule.
B. The central atom is Se, which has six electron groups around it.The electron-group geometry is octahedral. To facilitate thisgeometry, the Se atom's orbitals are hybridized as sp3d2 orbitals.Four of these hybrid orbitals contain one of Se's 6 valenceelectrons each, and these hybrid orbitals overlap with unhybridizedp orbitals of the four F atoms. The fifth and sixth hybrid orbitalsof Se contain lone pairs of electrons. There are 4 sigma bonds and0 pi bonds in this molecule.
C. The central atom is Se, which has four electron groups aroundit. The electron-group geometry is tetrahedral. To facilitate thisgeometry, the Se atom's orbitals are hybridized as sp3 orbitals.These hybrid orbitals overlap with unhybridized p orbitals of thefour F atoms. There are 4 sigma bonds and 0 pi bonds in thismolecule.
D. The central atom is Se, which has four electron groups aroundit, two single bonds and two double bonds. The electron-groupgeometry is tetrahedral. To facilitate this geometry, the Se atom'sorbitals are hybridized as sp3 orbitals. These hybrid orbitalscontain one electron each of Se's 6 valence electrons, and thesehybrid orbitals overlap with unhybridized p orbitals of the four Fatoms. The final 2 electrons of Se's 6 valence electrons reside inunhybridized p orbitals, which overlap with unhybridized p orbitalsof the F atoms. There are 4 sigma bonds and 2 pi bonds in thismolecule.