PV Work
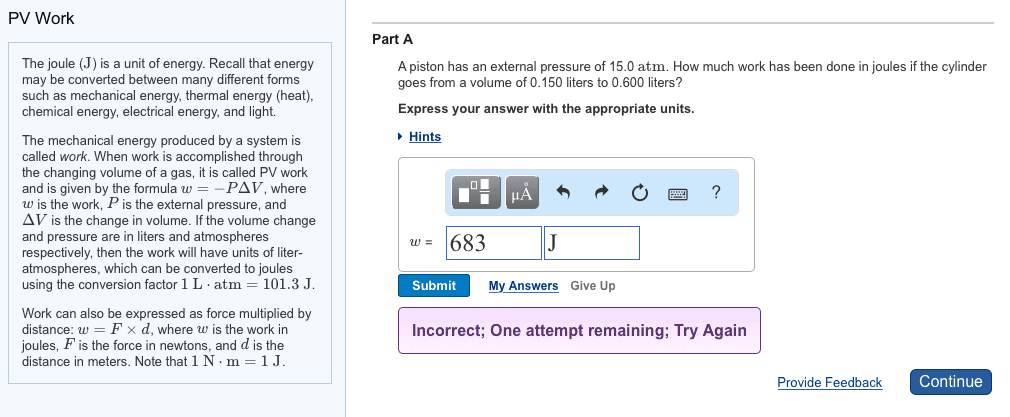
The joule (J) is a unit of energy. Recall that energy may be converted between many different forms such as mechanical energy, thermal energy (heat), chemical energy, electrical energy, and light. The mechanical energy produced by a system is called work. When work is accomplished through the changing volume of a gas, it is called PV work and is given by the formula w=âPÎV, where w is the work, P is the external pressure, and ÎV is the change in volume. If the volume change and pressure are in liters and atmospheres respectively, then the work will have units of liter-atmospheres, which can be converted to joules using the conversion factor 1 Lâ atm=101.3 J.
Work can also be expressed as force multiplied by distance: w=FÃd, where w is the work in joules, F is the force in newtons, and d is the distance in meters. Note that 1 Nâ m=1 J.
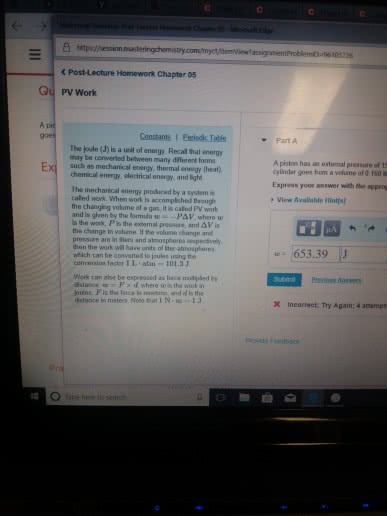
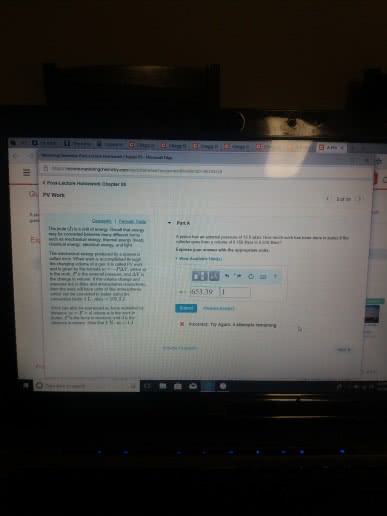
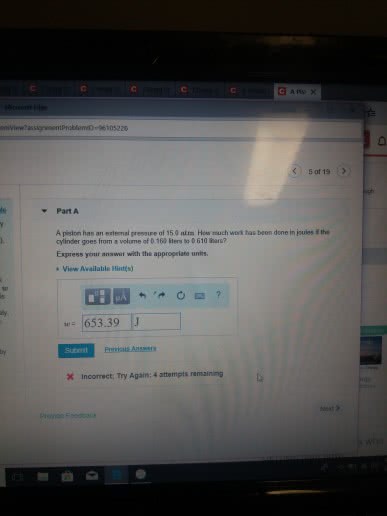
Part A
A piston has an external pressure of 9.00 atm. How much work has been done in joules if the cylinder goes from a volume of 0.160 liters to 0.550 liters?
Express your answer with the appropriate units.
Hints
w =
| PV Work The joule (J) is a unit of energy. Recall that energy may be converted between many different forms such as mechanical energy, thermal energy (heat), chemical energy, electrical energy, and light.The mechanical energy produced by a system is called work. When work is accomplished through the changing volume of a gas, it is called PV work and is given by the formula w=âPÎV, where w is the work, P is the external pressure, and ÎV is the change in volume. If the volume change and pressure are in liters and atmospheres respectively, then the work will have units of liter-atmospheres, which can be converted to joules using the conversion factor 1 Lâ atm=101.3 J. Work can also be expressed as force multiplied by distance: w=FÃd, where w is the work in joules, F is the force in newtons, and d is the distance in meters. Note that 1 Nâ m=1 J. | Part A A piston has an external pressure of 9.00 atm. How much work has been done in joules if the cylinder goes from a volume of 0.160 liters to 0.550 liters? Express your answer with the appropriate units. Hints
|