1
answer
0
watching
321
views
11 Nov 2019
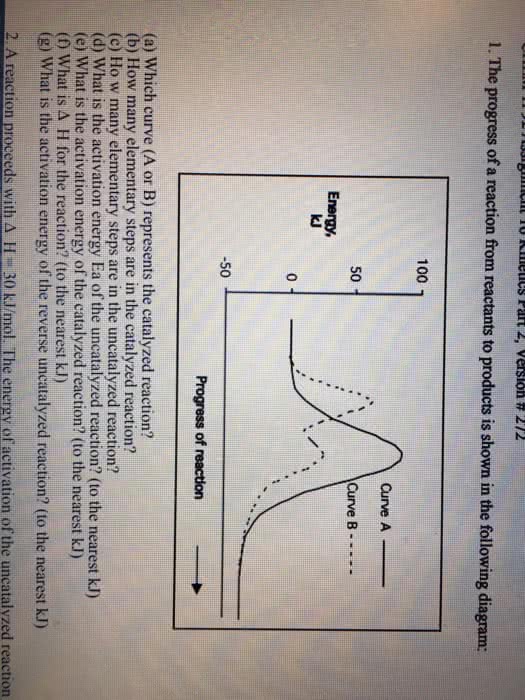
A reaction proceeds with â H = 30 kJ/mol. The energy of activation of the uncatalyzed reaction is 100 kJ/mol, whereas it is 40 kJ/mol for the catalyzed reaction. How many times faster is the catalyzed reaction than the uncatalyzed reaction at 25°C? Express your answer in scientific notation to two significant figures.
A reaction proceeds with â H = 30 kJ/mol. The energy of activation of the uncatalyzed reaction is 100 kJ/mol, whereas it is 40 kJ/mol for the catalyzed reaction. How many times faster is the catalyzed reaction than the uncatalyzed reaction at 25°C? Express your answer in scientific notation to two significant figures.
Lelia LubowitzLv2
12 Jan 2019