CH 302 Lecture Notes - Lecture 1: Weak Base, Ionic Compound, Sodium Chloride
Document Summary
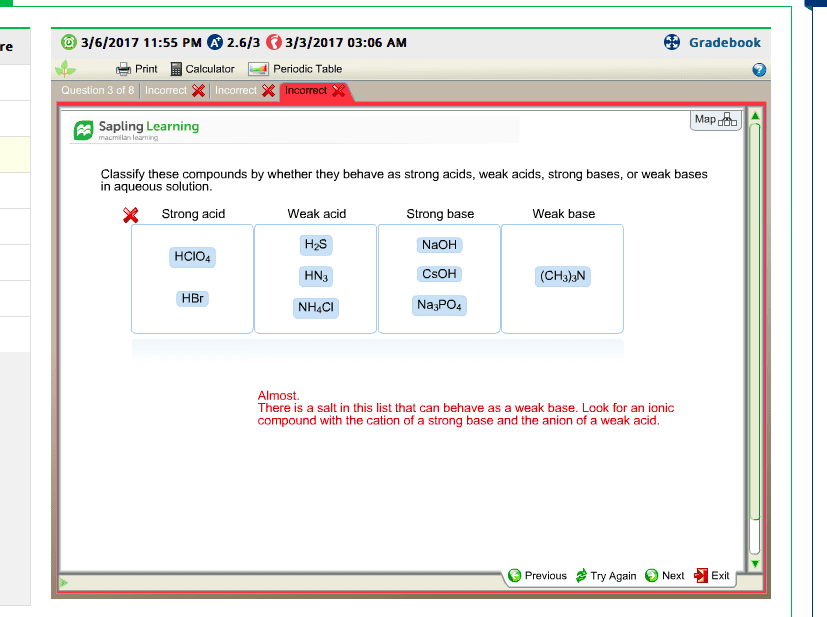
A salt is an ionic compound in which the anion is neither o2- nor oh-. A salt is formed in the reaction of an acid and a base. The cation part of the salt comes from the base and the anion part comes from the acid. Nacl: na+, the cation, comes from the base (naoh) and cl-, the anion comes from the acid (hcl). Salts can be classified based on the acid and the base from which they were formed. 1- salts of strong bases and strong acids. 2- salts of strong bases and weak acids. 3- salt of weak bases and strong acids. 4- salts of weak bases and weak acids. Based on their classification, when dissolved in water, salts can give neutral, acidic, or basic aqueous solutions. When we dissolve a salt of a strong base and a strong base in water the resulting solution is always neutral. When placed in water, solid nacl completely dissociates: