CHEM104 Lecture Notes - Lecture 3: Bond-Dissociation Energy, Cathode Ray, Bond Order
41 views5 pages
11 Apr 2016
School
Department
Course
Professor
Document Summary
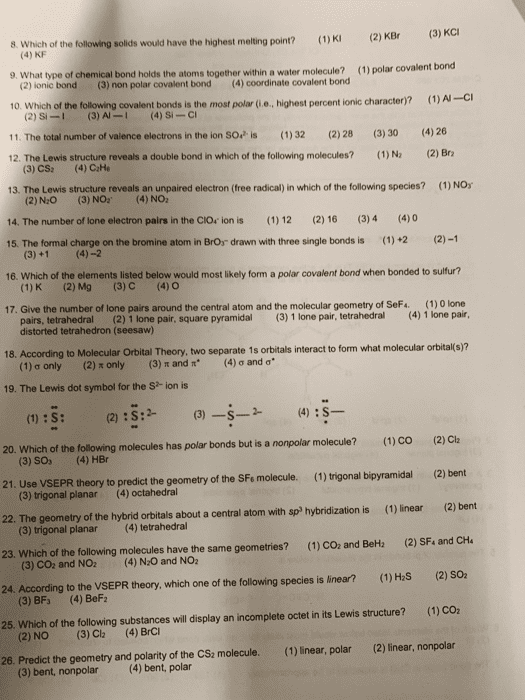
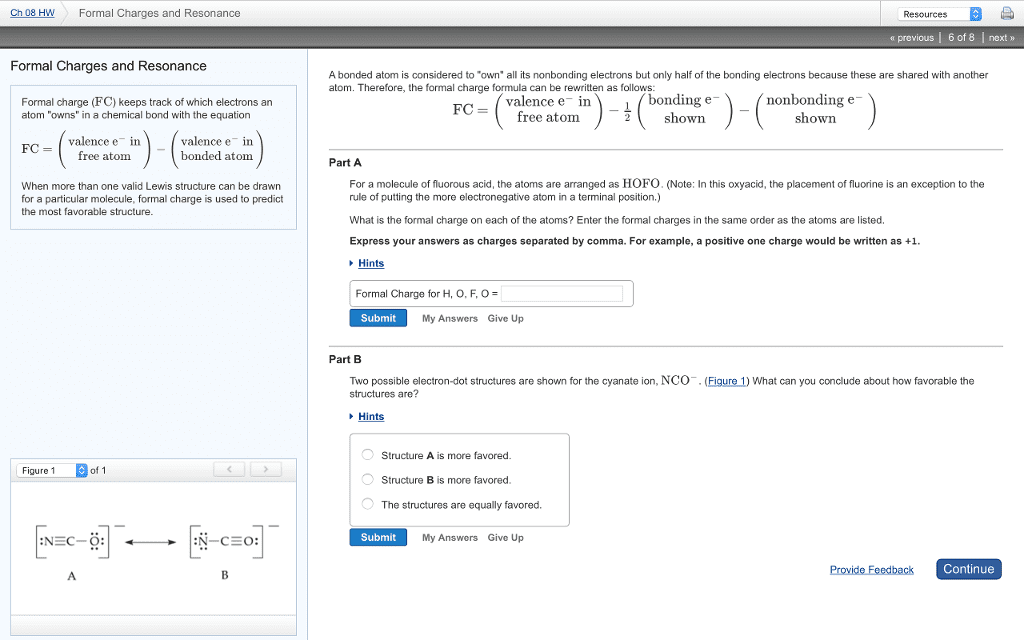
Molecular and electronic structure: electronic structure shows electrons and atoms, molecular structure shows only atoms but lp electrons on central atom still do influence molecular structure. They can look similar due to structure but aren"t the same. Polarity of a molecule: polarity results from unequal pull of exterior atoms on the central atom, the pull of the exterior atoms on central atom is the vector. Sum of all the bond dipoles (electronegaivity diference) Dipole-a pair of equal and oppositely charged or magneized poles separates by a distance. Nonpolar when charges are the same (only when they are same element) If the pull is balanced, then the molecule is nonpolar. If the pull is unbalanced, then the molecule is polar. Unbalanced pull of exterior atoms on the central atom results from. Lp electrons which don"t compensate the opposing bond dipoles. Diferent exterior atoms which form diferent strength bond dipoles (diferent electronegaivity diferences) Bond center atom to orbiing atoms (least electronegaive)
Get access
Grade+
$40 USD/m
Billed monthly

Homework Help
Study Guides
Textbook Solutions
Class Notes
Textbook Notes
Booster Class
10 Verified Answers
Class+
$30 USD/m
Billed monthly

Homework Help
Study Guides
Textbook Solutions
Class Notes
Textbook Notes
Booster Class
7 Verified Answers
Related textbook solutions
Chemistry: Structure and Properties
2 Edition,
Tro
ISBN: 9780134293936
Basic Chemistry
5 Edition,
Timberlake
ISBN: 9780134138046
Principles of Chemistry Molecular Approach
4th Edition,
Tro
ISBN: 9780134112831
Chemistry: Structure and Properties
2nd Edition,
Tro
ISBN: 9780134293936
Principles of Chemistry Molecular Approach
3rd Edition, 2014
Tro
ISBN: 9780321971944
Chemistry: A Molecular Approach
3rd Edition,
Tro
ISBN: 9780321809247
Chemistry: A Molecular Approach
5th Edition,
Tro
ISBN: 9780134874371
Principles of Chemistry: A Molecular Approach
4th Edition,
Tro
ISBN: 9780134895741
Chemistry: The Central Science
14th Edition, 2017
Brown
ISBN: 9780134414232