CHE 202 Lecture Notes - Lecture 12: Trigonal Planar Molecular Geometry, Bond Order, Covalent Bond
Document Summary
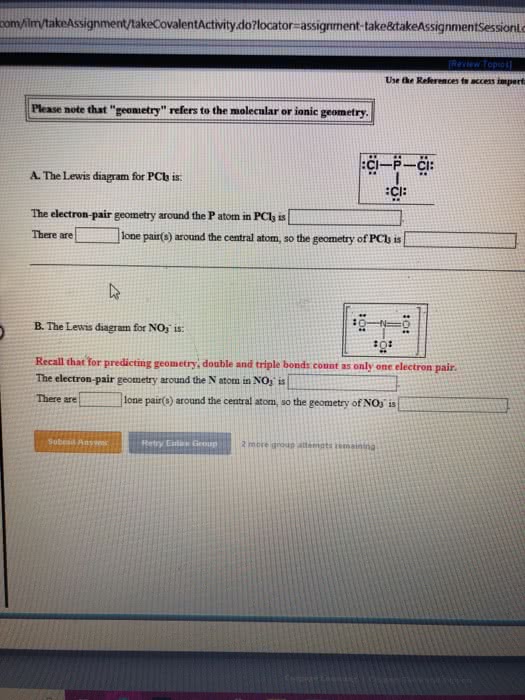
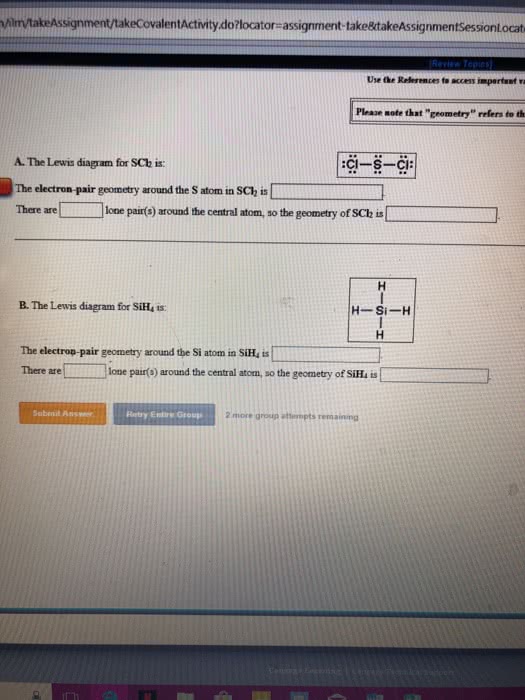
Ionic non - metal t metal covalent non - metals. Electron geometry ( no lp) sp linear spas trigonal planar. Molecular geometry (lp ) bonds - lone pairs z - i bent. Cadore : never bent , when triple bond ( linear ) Cid is the total number of electrons c. d. Label under " is " the element calculating bond order : Draw first carbon in front (ee - ee) ; draw the molecules left to right . Butane : n see - butyl : r-4 tert-butyl : r -e. Naming structures : number, write in alphabetical order (ignore di. , cyclo . in lowest sequence , then alphabetical but do not. Constitutional isomer same formula , but structure is altered cis ( trans or geometric isomer flipped organization at carbon . Melting and boiling points ( increases: hydrogen bonds, number of carbons cbp, triple double bonds.