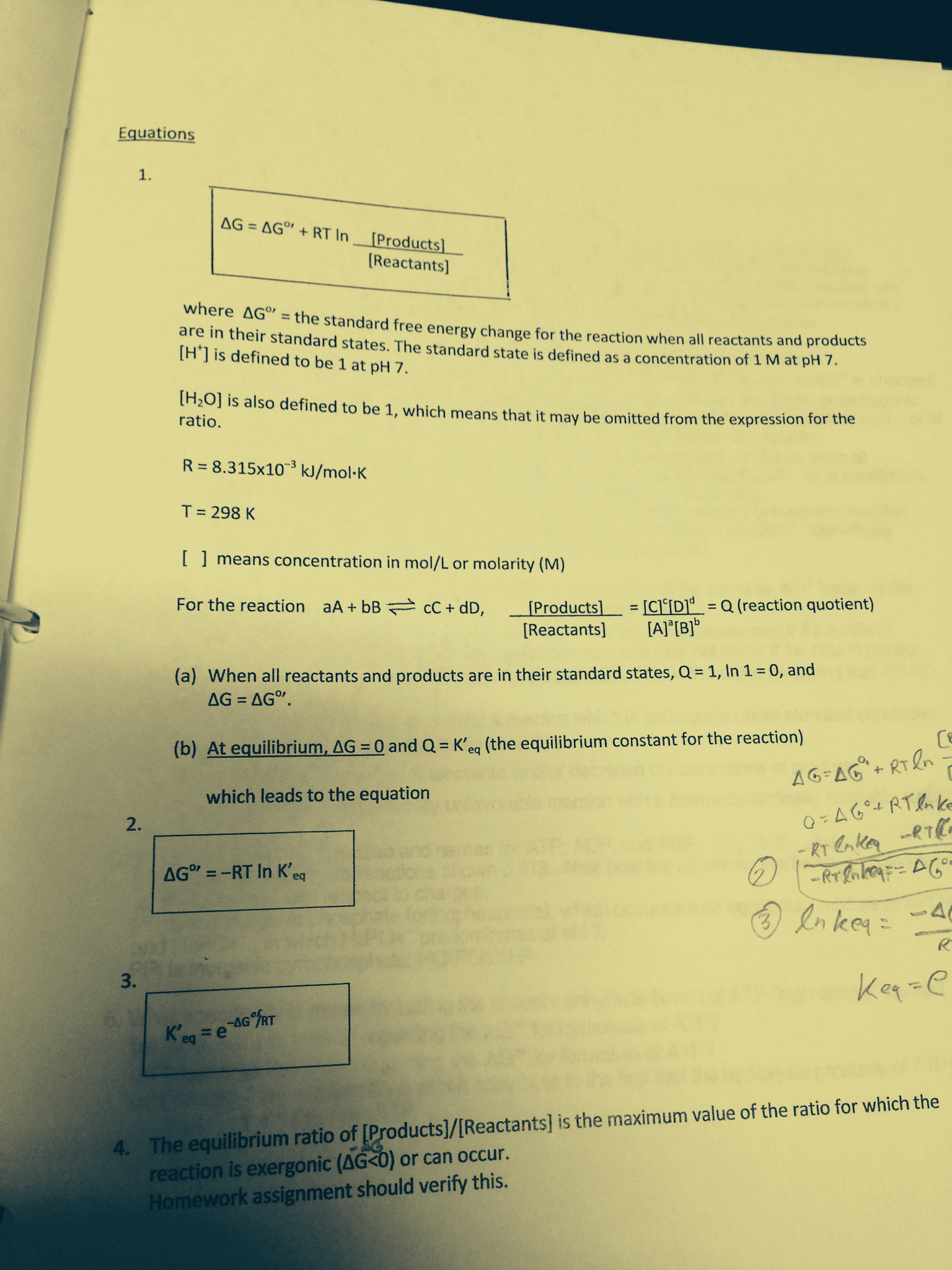CHM 145 Lecture Notes - Lecture 21: Boiling Point, Chemical Equation, Jmol
41 views5 pages
24 Mar 2017
School
Department
Course
Professor
Document Summary
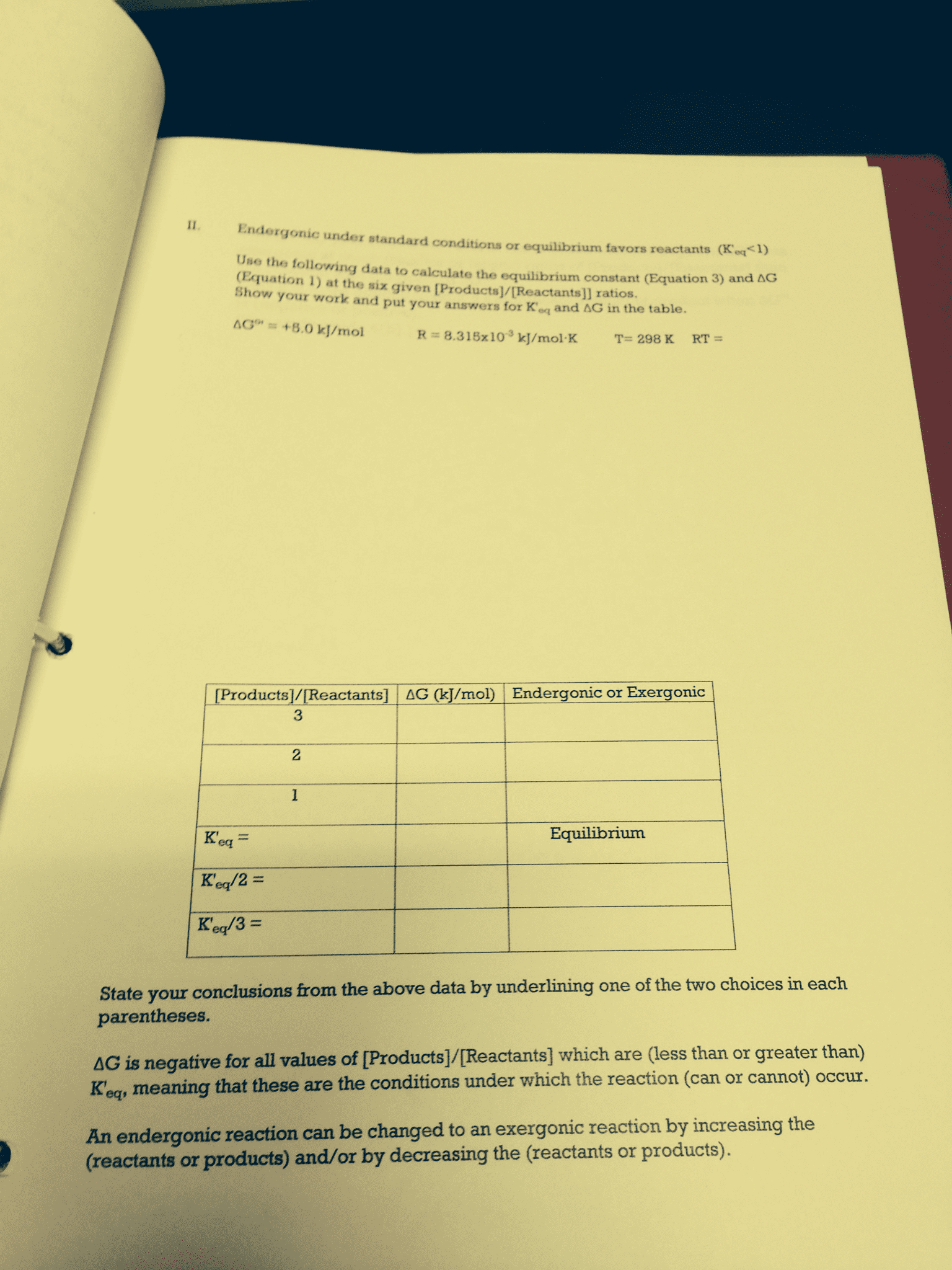
G = g o + rt ln q: under standard conditions , all concentration values are 1, q=1, rt ln q = 0, and g = g o. Equilibrium and thermodynamics: at equilibrium, g=0 and q=k: Thus, g o = rt ln k or k = e go /rt. Note: this equation can only be applied at t=298 k! G o = rt ln k: if k is large (>> 1), g o becomes a large negative value ( spontaneous = large k) Spontaneous = product-favored reaction: if k is small (<< 1), go becomes a large positive value ( nonspontaneous = small k) Nonspontaneous = reactant-favored reaction: if k 1, go 0 & you are near equilibrium equilibrium lies between reactants & products, and significant amounts of each are present. The point where free energy is at a minimum defines the composition of a system at equilibrium under standard conditions!
Get access
Grade+
$40 USD/m
Billed monthly

Homework Help
Study Guides
Textbook Solutions
Class Notes
Textbook Notes
Booster Class
10 Verified Answers
Class+
$30 USD/m
Billed monthly

Homework Help
Study Guides
Textbook Solutions
Class Notes
Textbook Notes
Booster Class
7 Verified Answers