CHEM 411 Lecture Notes - Lecture 4: Weak Base, Conjugate Acid, Acid Dissociation Constant
Document Summary
Get access


Related textbook solutions
Chemistry: Structure and Properties
Basic Chemistry
Principles of Chemistry Molecular Approach
Principles of Chemistry Molecular Approach
Chemistry: Structure and Properties
Chemistry: A Molecular Approach
Chemistry: A Molecular Approach
Principles of Chemistry: A Molecular Approach
Chemistry: The Central Science
Related Documents
Related Questions
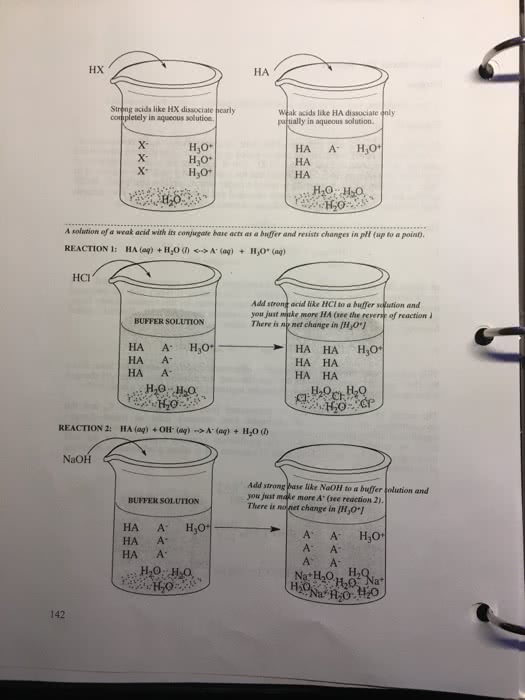
For the dissociation reaction of a weak acid in water,
HA(aq)+H2O(l)âH3O+(aq)+Aâ(aq)
the equilibrium constant is the acid-dissociation constant, Ka, and takes the form
Ka=[H3O+][Aâ][HA]
Weak bases accept a proton from water to give the conjugate acid and OHâ ions:
B(aq)+H2O(l)âBH+(aq)+OHâ(aq)
The equilibrium constant Kb is called the base-dissociation constant and can be found by the formula
Kb=[BH+][OHâ][B]
When solving equilibrium-based expression, it is often helpful to keep track of changing concentrations is through what is often called an I.C.E table, where I. stands for Initial Concentration, C. stands for Change, and E. stands for Equilibrium Concentration. To create such a table, write the reaction across the top creating the columns, and the rows I.C.E on the left-hand side.
Initial (M)Change (M)Equilibrium (M)A+ BâAB
Part A
Aspirin (acetylsalicylic acid, C9H8O4) is a weak monoprotic acid. To determine its acid-dissociation constant, a student dissolved 2.00 g of aspirin in 0.600 L of water and measured the pH. What was the Ka value calculated by the student if the pH of the solution was 2.60?
Express your answer numerically using two significant figures.
Hints
| |||||||
| Ka = | |||||||
SubmitMy AnswersGive Up
Incorrect; Try Again; 3 attempts remaining
You used the initial concentration of aspirin in the Ka expression. Instead, you need to use the equilibrium concentration. Consider that the amount of aspirin that reacted is equal to the amount of H3O+ produced. How much is left over?
Part B
A 0.100 M solution of ethylamine (C2H5NH2) has a pH of 11.87. Calculate the Kb for ethylamine.
Express your answer numerically using two significant figures.
Hints
| | |||
| Kb = |
Part A - Calculate the molar solubility in water
Mg(OH)2 is a sparingly soluble salt with a solubility product, Ksp, of 5.61Ã10?11. It is used to control the pH and provide nutrients in the biological (microbial) treatment of municipal wastewater streams.
Based on the given value of the Ksp, what is the molar solubility of Mg(OH)2 in pure H2O?
Express your answer with the appropriate units.
| molar solubility = |
2.41Ã10?4 M |
Calculate the molar solubility in NaOH
Based on the given value of the Ksp, what is the molar solubility of Mg(OH)2 in 0.120 M NaOH?
Express your answer with the appropriate units.
| molar solubility = |
3.90Ã10?9 M |
Part C: Calculate how many times more soluble Mg(OH)2 is in pure water
Based on the given value of the Ksp, calculate the ratio of solubility of Mg(OH)2 dissolved in pure H2O to Mg(OH)2 dissolved in a 0.120 M NaOH solution.
A buffer is a mixed solution of a weak acid or base, combined with its conjugate. Note that this can be understood essentially as a common-ion problem: The conjugate is a common ion added to an equilibrium system of a weak acid or base. The addition of the conjugate shifts the equilibrium of the system to relieve the stress of the added concentration of the common ion. In a solution consisting of a weak acid or base, the equilibrium shift also results in a pH shift of the system.
It is the presence of the common ion in the system that results in buffering behavior, because both added H+ or OH? ions can be neutralized.
Part D
What is the pH change of a 0.240 M solution of citric acid (pKa=4.77) if citrate is added to a concentration of 0.175 M with no change in volume?