Chemistry 1027A/B Lecture Notes - Lecture 1: Organic Chemistry, Orbital Hybridisation, Covalent Bond
Document Summary
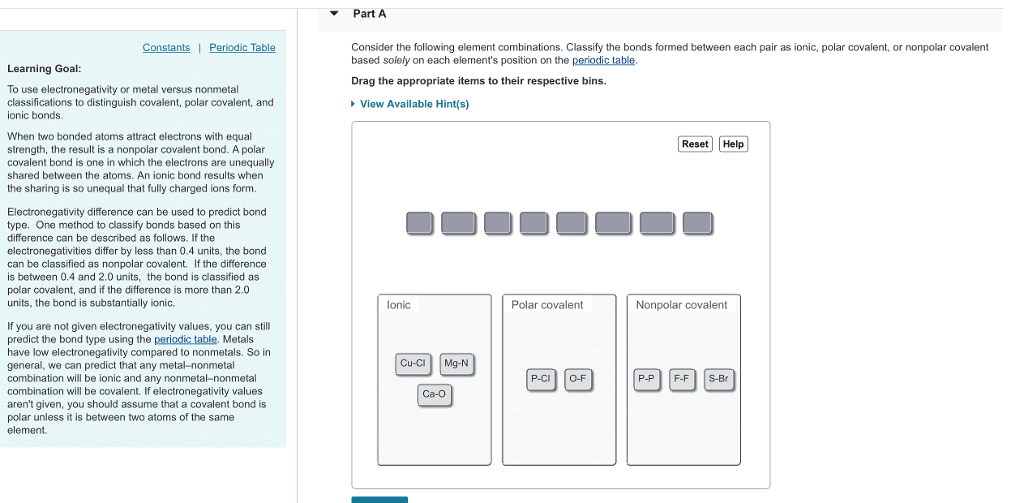
Organic chemistry key concepts: topic 0 (usselman version) The number in the upper right of a given atom on the periodic table is the number of valence electrons (the number of unpaired electrons in the valence shell). The lower right denotes the atom"s electronegativity value. Electronegativity reflects an atom"s ability to attract a shared pair of electrons to itself. Electronegativity values increase up and to the right of the periodic table. Polar bonds result from the unequal sharing of electrons between unlike atoms with electronegativity differences < 1. 9, but > 0. 5. As a note, rank hydrogen as having relatively the same electronegativity value as carbon. Sometimes the dipoles of polar bonds cancel each other out, so the molecule itself is non-polar (has no net-dipole moment). The 3d structure of a molecule needs to be known before its overall polarity can be recognized. The most common way of depicting 3-dimensional structures is the dot-line-wedge symbolism.