CHEM102 Lecture Notes - Lecture 4: Reaction Rate, Reaction Rate Constant, Rate Equation

32
CHEM102 Full Course Notes
Verified Note
32 documents
Document Summary
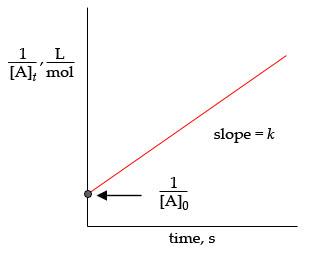
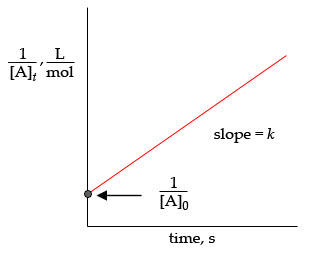
Plot of reactant concentration [a] against time for different order of reactions. Rate = k[a] s-1 ln[a]t = - kt + ln[a]o ln[a] vs time. If the rate constant is 7. 30 x 10-4 s-1, calculate the half- life of this reaction. The half-life of a first order reaction is given by: ln 2. Kt = - 7. 30 x 10-4 s-1 x 500. 0 s = - 0. 365. Percentage of h2o2 reacted = 100 - 69. 4 = 30. 6% A reaction is 50% complete in 30. 0 min. How long does it take to have the reaction 75 % complete if it is (a) a first order reaction and (b) a second order reaction? ln 2 (a) Half-life for first order reaction is given by: = 30. 0 min ln 2. For 75% complete, the fraction remaining is (100 75) / 100 = 0. 25. = 0. 25 = e 0. 0231 x t. = 60. 0 min ln 0. 25 = - 0. 0231 t ln 0. 25 t = -