BIOCH200 Lecture Notes - Lecture 14: Competitive Inhibition, Dissociation Constant, Allosteric Regulation
46 views2 pages
16 Apr 2018
School
Department
Course
Professor
Document Summary
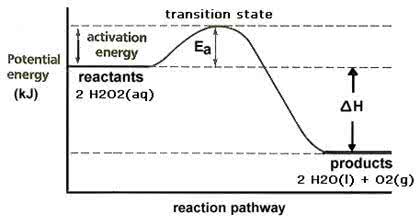
01. 03. 18 lec 14 delta g tells us about spontaneity. Its magnitude tells us how spontaneous the reaction is, how much heat will be given off. Transition state is the highest free energy state. More positive the delta g more slow the reaction more activation energy. , more energy put in for collision and for the right orientation of collisions. They just affect activation energy not free energy. Enzymes lower the free energy of transition state. In 1 degree structure residues are far apart. In 3 degree structure residues can come close. Affinity vs specificity (how tightly binds vs a binds, not b) In case of bpg, look for positively charged groups. Any enzyme that uses atp (except those in muscle contraction) does not want water. This is because phosphate will be released which means energy will be released. Advantages of removing water: water hydrolyses, forms h bonds, hydrophobic molecules will have a shell of water around.
Get access
Grade+
$40 USD/m
Billed monthly

Homework Help
Study Guides
Textbook Solutions
Class Notes
Textbook Notes
Booster Class
10 Verified Answers
Class+
$30 USD/m
Billed monthly

Homework Help
Study Guides
Textbook Solutions
Class Notes
Textbook Notes
Booster Class
7 Verified Answers