kyojuro
Polytechnic University of the Philippines
2 Followers
0 Following
2 Helped
umai
kyojuroLv10
3 Jul 2022
Answer:
kyojuroLv10
3 Jul 2022
Answer: a) At first HCl solution added , AgCl (white ppt) precipitated then SO...
kyojuroLv10
3 Jul 2022
Answer:
kyojuroLv10
3 Jul 2022
Answer: Here we found 7.7 moles of ATP produced.
kyojuroLv10
3 Jul 2022
Answer:
kyojuroLv10
3 Jul 2022
Answer:
kyojuroLv10
3 Jul 2022
Answer: False
kyojuroLv10
3 Jul 2022
Answer: The total % of the compound is assumed to be 100%. The 20% of the comp...
kyojuroLv10
3 Jul 2022
Answer: 12) [OH-] = 3.25*10^-8 M , pH = -log[H+] , pH + pOH = 14 , pOH = -log[...
kyojuroLv10
3 Jul 2022
Answer:
kyojuroLv10
3 Jul 2022
Answer:
kyojuroLv10
3 Jul 2022
Answer: 9) 0.02531g =25.31kg it is false because, 1 gram = 0.001kg So, to calc...
kyojuroLv10
3 Jul 2022
Answer:
kyojuroLv10
3 Jul 2022
Answer:
kyojuroLv10
3 Jul 2022
Answer: Given that Total pressure in inhaled air =total pressure in exhaled ai...
kyojuroLv10
3 Jul 2022
Answer: Answer: Pressure, P = 3.179 atm Explanation: According to the Ideal ga...
kyojuroLv10
3 Jul 2022
Answer:
kyojuroLv10
3 Jul 2022
Answer:
kyojuroLv10
3 Jul 2022
Answer: Answer- - 22.5 mL Miniscus of liquid level is between mark 22 and 2...
kyojuroLv10
3 Jul 2022
Answer: The dissociation of acid can be increased by adding H2O The addition o...
kyojuroLv10
3 Jul 2022
Answer: a) For the NO2- Lewis structure, calculate the total number of valence...
kyojuroLv10
3 Jul 2022
Answer:
kyojuroLv10
3 Jul 2022
Answer:
kyojuroLv10
3 Jul 2022
Answer: We know that, dilution calculations can be performed using the formula...
kyojuroLv10
3 Jul 2022
Answer:
kyojuroLv10
3 Jul 2022
Answer:
kyojuroLv10
3 Jul 2022
Answer:
kyojuroLv10
3 Jul 2022
Answer: This is an awesome problem of analytical chemistry.
kyojuroLv10
3 Jul 2022
Answer:
kyojuroLv10
3 Jul 2022
Answer:
kyojuroLv10
3 Jul 2022
Answer:
kyojuroLv10
3 Jul 2022
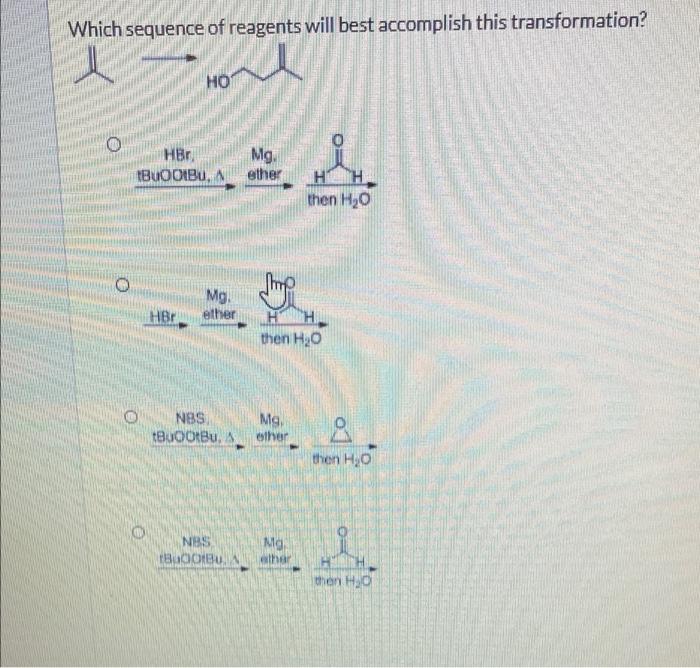
Answer: Expert Q&A Find solutions to your homework Search Question Show tr...
kyojuroLv10
3 Jul 2022
Answer: Entropy, enthalpy and Gibbs free energy are important thermodynamic pa...
kyojuroLv10
3 Jul 2022
Answer: 1) We have the following reaction: Br2 + 2I- => 2Br- + I2 We determ...
kyojuroLv10
3 Jul 2022
Answer: Loss of electrons is oxidation while gain of electrons is reduction. F...
kyojuroLv10
3 Jul 2022
Answer:
kyojuroLv10
3 Jul 2022
Answer:
kyojuroLv10
3 Jul 2022
Answer:
kyojuroLv10
3 Jul 2022
Answer:
kyojuroLv10
3 Jul 2022
Answer: